CQ I-1
How is headache classified and diagnosed?
Recommendation
Headache should be classified and diagnosed according to the International Classification of Headache Disorders 3rd edition (beta version).
| Grade A |
Background and Objective
In 2004, the International Headache Society (IHS) revised the first edition of the IHS guideline for the first time in 15 years, incorporating the latest advances in research, evidence and criticisms. The resulting document, International Classification of Headache Disorders 2nd Edition (ICHD-2) was published in Cephalalgia.1) In the same year, the ICHD-2 was translated into Japanese and published.2) From 2004, headache should be classified and diagnosed in accordance with the ICHD-2.
The first recorded classification of headache was by Aretaeus (a physician born in 81 BC) of Cappadacia in the present day Turkey, who classified headaches into cephalalgia, cephalea, and heterocrania.3)-5) Heterocrania was described as “half head” headache, which is equivalent to migraine in the present day classification.
The first consensus-orientated headache classification in history was the classification by the Ad Hoc Committee on Classification of Headache of the American Neurological Association (Ad Hoc classification) published in 1962.6) In this classification, headache was classified into 15 types, but no diagnostic criteria were included.
In 1988, the Headache Classification Committee of the International Headache Society chaired by Olesen proposed the first international classification of headache disorders (IHS Classification, 1st edition, 1988).7) The IHS Classification 1st edition first classified headache into 13 items, and further subdivided into 165 headache types. For each subtype, operational criteria were described. Since the IHS Classification 1st edition placed greater weight on the nervous system rather than the vascular system as the mechanism of migraine development, the concept of vascular headache was abandoned. Migraine and cluster headache were classified independently, and muscle contraction headache was renamed tension-type headache.
When the IHS Classification 1st edition was tested on 740 persons, only 2 persons (0.3%) had unclassifiable headache, verifying that the classification covers the vast majority of headaches.5) The consistency, reproducibility and reliability of the operational criteria in the IHS Classification 1st edition were validated by clinical evaluations.8)9)
Several commentaries on the ICHD-2 have been published.2)5)10)-12)
Due to clinical necessity, an appendix for chronic migraine and medication overuse headache (MOH) were added in 2006.13)14) Furthermore, revision of the diagnostic criteria for secondary headache was proposed.15)16) The Classification Committee of the International Headache Society has been preparing for the publication of the third edition of ICHD. The ICHD Third Edition (beta version) (ICHD-3beta) was published in 2013.17)
Comments and Evidence
Headache classification according to the ICHD-3beta17)
The ICHD-3beta is composed of the following three parts
Part one The primary headaches: 4 types (57 subtypes)
Part two The secondary headaches: 8 types (117 subtypes)
Part three Painful cranial neuropathies, other facial pains and other headaches: 2 types (29 subtypes) supplement (17 subtypes)
Appendix (40 subtypes)
Broad Classification of Headache
• Part one: The primary headaches
1. Migraine
2. Tension-type headache (TTH)
3. Trigeminal autonomic cephalalgias (TACs)
4. Other primary headache disorders
• Part two: The secondary headaches
5. Headache attributed to trauma or injury to the head and/or neck
6. Headache attributed to cranial or cervical vascular disorder
7. Headache attributed to non-vascular intracranial disorder
8. Headache attributed to a substance or its withdrawal
9. Headache attributed to infection
10. Headache attributed to disorder of homoeostasis
11. Headache or facial pain attributed to disorder of the cranium, neck, eyes, ears, nose, sinuses, teeth, mouth or other facial or cranial structure
12. Headache attributed to psychiatric disorder
• Part three: Painful cranial neuropathies, other facial and other headaches
13. Painful cranial neuropathies and other facial pains
14. Other headache disorders
• Appendix
Notes
• While the 1st edition had 13 categories, the International Classification of Headache Disorders 2nd Edition (ICHD-2) has an added category “12. Headache attributed to psychiatric disorder” and thus a total of 14 categories.
• The ICHD-2 is an indispensable reference for the treatment and diagnosis, research, and education of headache disorders.
• At least, physicians should acquire a good knowledge of migraine (migraine without aura and migraine with aura), tension-type headache, cluster headache, and medication overuse migraine.
• Although the classification was revised by consolidating a vast volume of evidence on headache accumulated during 15 years since publication of the 1st edition, the basic policy is based on that of the 1st edition.
• Headache is classified based on the hierarchical classification system into group → type → subtype → sub-form. According to this system, each headache is coded in four digits. However, in clinical practice, classification up to two digits is sufficient.
• The following new headache disorders have been added: 1.5.1 Chronic migraine, 4.5 Hypnic headache, 4.6 Primary thunderclap headache, and 4.7 Hemicrania continua.
• For some headaches, the classification code was changed (for example; 1.3 Ophthalmoplegic migraine was moved to 13.17 Ophthalmoplegic migraine).
• Reflecting new concept of pathophysiology, the names of some headaches were changed [for example; trigeminal-autonomic cephalalgias (TAC)].
• In the Japanese translation of the ICHD-2, some translated terms were revised, such as “Migraine not associated with aura” to “Migraine without aura”.
• This classification is compiled in the same format as the World Health Organization (WHO) International Classification of Disease, and is compatible with the International Classification of Diseases, 10th revision: Neurological Adaptation (ICD-10NA).
• Soon after the publication of ICHD-2, the necessity to revise the diagnostic criteria for MOH was pointed out, and they were revised in March 2004. The major changes were (1) deletion of the characteristics of headache described in the subform of medication overuse headache; (2) addition of a new subform “8.2.6 Medication overuse headache attributed to combination of acute medications”. These two changes have been incorporated in the Japanese edition of the ICHD-2.2)18)
• The Japanese edition of ICHD-2 was published in 2004 in the official journal of the Japanese Headache Society.2) A book has since been published which detailed the errata of typographical errors and subsequent changes.19)
• An important point of the 2006 revision is that MOH can be diagnosed when there is misuse of medication, and the condition of headache improvement after drug discontinuation is no longer needed. For chronic migraine, while it was required in the past that the headache fulfills at least the diagnostic criteria for migraine without aura, at present it is not necessary that the headache shows the characteristics of migraine.16)
• The current diagnostic criterion D for secondary headaches is “Headache is greatly reduced or resolved within 3 months (this may be shorter for some disorders) after successful treatment or spontaneous remission of the causative disorder”. According to this, the headache should disappear completely or improve markedly after the causative disease is cured. However, some causative diseases cannot be cured and as a result headache is perpetuated. In the draft revision for ICHD-3, the diagnostic criterion C is revised substantially to better demonstrate the evidence of causal relationship. Fulfilment of at least two of five sub-criteria is required. In other words, while the current criterion C focuses only on the temporal relation of the development of headache with the onset of causative disorder, the new proposal has additional items: (C1) headache has developed in temporal relation to the onset of the causative disorder; (C2) headache has worsened in parallel with the causative disorder; (C3) headache has improved in parallel with the presumed causative disorder; (C4) headache has characteristics typical for the causative disorder; (C5) other evidence exists of causation. Moreover, for criterion D, while the current required evidence is resolution or greatly reduced of headache by cure of the causative disorder, the new proposal abolishes this and added “not better accounted for by other diagnosis”.15)16)
• The Japanese edition of ICHD-3beta was published in 2014.20)
Major References
• Commentaries on International Classification of Headache Disorders 2nd Edition (ICHD-2)2)5)10)-12)
• Original ICHD-3beta (in English). URL; http://www.ihs-classification.org/_downloads/mixed/International-Headache-Classification-III-ICHD-III-2013-Beta.pdf#search=%27ICHD3%27
• References
1) Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24(Suppl 1): 9-160.
2) The Headache Classification Committee of International Headache Society: International Classification of Headache Disorders 2nd Edition (ICHD-II). Japanese Journal of Headache 2004; 31(1): 13-188. (In Japanese)
3) Isler H: Headache classification prior to the Ad Hoc criteria. Cephalalgia 1993 ; 13(Suppl 12): 9-10.
4) Manaka N: History of headache research. Advances in Neurological Sciences 2002; 46(3): 331-340. (In Japanese)
5) Gladstone JP, Dodick DW: From hemicrania lunaris to hemicrania continua: an overview of the revised International Classification of Headache Disorders. Headache 2004; 44(7): 692-705.
6) The Ad Hoc Committee on Classification of Headache: Classification of Headache. Arch Neurol 1962; 6: 173-176.
7) Headache Classification Committee of the International Headache Society: Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8(Suppl 7): 1-96.
8) Granella F, D’Alessandro R, Manzoni GC, Cerbo R, Colucci D’Amato C, Pini LA, et al : International Headache Society classification: interobserver reliability in the diagnosis of primary headaches. Cephalalgia 1994; 14(1): 16-20.
9) Leone M, Filippini G, D’Amico D, Farinotti M, Bussone G: Assessment of International Headache Society diagnostic criteria: a reliability study. Cephalalgia 1994; 14(4): 280-284.
10) Sakai F: [Latest topic on headache] New international classification. No To Shinkei 2004; 56(8): 639-643. (In Japanese)
11) Fujiki N: [Cutting edge of headache care, for better headache care] New international diagnostic criteria for headache. Current Therapy 2004; 22(10): 979-982. (In Japanese)
12) Sakai F (ed.), Manaka S: International Classification of Headache Disorders (ICHD-II): Primary headaches, secondary headaches. ABC of New Diagnosis and Treatment (21) Neurology 2: Headache 2004: 9-15. (In Japanese)
13) Headache Classification Committee, Olesen J, Bousser MG, Diener HC, et al: New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 2006; 26(6): 742-746.
14) Takeshima T, Manaka S, Igarashi H, Hirata K, Sakai F, International Headache Classification Promotion Committee of Japanese Headache Society: On the addition of appendix for chronic migraine and medication overuse headache. Japanese Journal of Headache 2007; 34(2): 192-193. (In Japanese)
15) Olesen J, Steiner T, Bousser MG, Diener HC, Dodick D, First MB, Goadsby PJ, Gobel H, Lainez MJ, Lipton RB, Nappi G, Sakai F, Schoenen J, Silberstein SD: Proposals for new standardized general diagnostic criteria for the secondary headaches. Cephalalgia 2009; 29(12): 1331-1336.
16) Takeshima T, Manaka S, Igashira H, Hirata K, Yamane K, Sakai F, New International Headache Classification and Promotion Committee of Japanese Headache Society: On the proposed revision of the diagnostic criteria for secondary headaches of the International Classification of Headache Disorders 2nd Edition/(ICHD-II). Japanese Journal of Headache 2010; 36(3): 235-238. (In Japanese)
17) Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33(9): 629-808.
18) Igashira H, Manaka S: Revised diagnostic criteria for “8.2 Medication overuse headache” in the International Classification of Headache Disorders 2nd Edition first revision (ICHD-II R1) — Difference from the Japanese Edition of the International Classification of Headache Disorders 2nd Edition. Japanese Journal of Headache 2006; 33(1): 26-29. (In Japanese)
19) The Headache Classification Committee of the International Headache Society (author), International Headache Classification Promotion Committee of Japanese Headache Society (translator): Japanese Edition of the International Classification of Headache Disorders 2nd Edition. Igakushoin, 2007. (In Japanese)
20) The Headache Classification Committee of the International Headache Society (author), International Headache Classification Promotion Committee of Japanese Headache Society (translator): Japanese Edition of the International Classification of Headache Disorders 3rd edition (beta version). Igakushoin, 2014. (In Japanese)
• Search terms and secondary sources
• Search database: Ichushi Web for articles published in Japan (2012/5/28)
classification of headache 58
headache classfication 118 (headache/TH or headache/AL) and (classification/TH or classification/AL) 798
• Search database: PubMed (2012/5/28)
classification of headache 3085
international classification of headache 1030
headache disorders/*classification 889
• Database used: Ichushi Web for articles published in Japan (2012/5/28)
(headache /TH or headache /AL) and diagnostic criteria /AL 242
• Database used: PubMed (2012/5/28)
headache/diagnostic criteria 3107
headache/*classification/*diagnosis 449
CQ I-2
How are primary headaches and secondary headaches differentiated?
Recommendation
Secondary headache should be suspected for the following: (1) headache with sudden onset, (2) headache never experienced before, (3) headache different from the customary headache, (4) headache that has increased in frequency and intensity, (5) headache begins after age 50, (6) headache with neurological deficit, (7) headache in a patient with cancer or immunodeficiency, (8) headache in a patient with psychiatric symptoms, and (9) headache in a patient with fever, neck stiffness or meningeal irritation. Intensive investigations are required.
| Grade A |
Background and Objective
Secondary headaches are headaches that develop due to some disorders, intracranial or otherwise, that cause the headache. In the International Classification of Headache Disorders 3rd Edition beta version (ICHD-3beta), the secondary headaches are coded under 5. “Headache attributed to trauma or injury to the head and/or neck”, 6. “Headache attributed to cranial or cervical vascular disorder”, 7. “Headache attributed to non-vascular intracranial disorder”, 8. “Headache attributed to a substance or its withdrawal”, 9. “Headache attributed to infection”, 10. “Headache attributed to disorder of homoeostasis”, 11. “Headache or facial pain attributed to disorder of the cranium, neck, eyes, ears, nose, sinuses, teeth, mouth or other facial or cranial structures”, and 12. “Headache attributed to psychiatric disorder”, and further subdivided into subtypes.1)2) There was an issue in the International Classification of Headache Disorders Second Edition regarding the classification and diagnosis of secondary headaches; which is, secondary headache cannot be diagnosed definitively if headache does not resolve after treatment.5) To address this issue, novel general diagnostic criteria for secondary headaches were proposed as a part of the revision task towards the publication of ICHD-3beta. As a result revision was adopted in ICHD-3beta.
Diverse disorders can cause secondary headaches, and some could be life-threatening. Therefore, careful examination is required. The phrase “Primary or secondary headache or both” is repeatedly discussed throughout the ICHD-3beta.1)2) The most important point in clinical care is that among the large number of disorders that may cause secondary headaches, do not miss the “headache for which a misdiagnosis will threaten life”.
Comments and Evidence
The diagnostic criterion D of ICHD-2 for secondary headaches states “Headache is greatly reduced or resolves within 3 months (this may be shorter for some disorders) after successful treatment or spontaneous remission of the causative disorder”. 5) According to this criterion, a diagnosis requires that the headache disappears completely or improves markedly after the causative disease is cured. However, some causative diseases cannot be cured, and as a result headache may be perpetuated. To address this issue, general diagnostic criteria for secondary headaches are proposed in ICHD-3beta,3)4) and they are presented below.
A. Any headache fulfilling criterion C
B. Another disorder scientifically documented to be able to cause headache has been diagnosed
C. Evidence of causation demonstrated by at least two of the following:
1. headache has developed in temporal relation to the onset of the presumed causative disorder
2. one or both of the following:
a) headache has significantly worsened in parallel with worsening of the presumed causative disorder
b) headache has significantly improved in parallel with improvement of the presumed causative disorder
3. headache has characteristics typical for the causative disorder
4. other evidence exists of causation
D. Not better accounted for by another ICHD-3 diagnosis.
Currently, the diagnostic criteria for each of the secondary headaches are being revised in line with the above general criteria.
First of all, differentiation between the primary headaches and the secondary headaches is important. The features that lead to a suspicion of secondary headache include “headache with sudden onset”, “headache never experienced before”, “headache different from the customary headache”, and “headache that tends to worsen”. The probability of secondary headache has to be considered for headaches that begin after age 50; headaches associated with neurological symptoms such as paralysis or abnormal visual acuity or visual field, change in consciousness level, and seizure; headaches associated with fever, rash, or neck stiffness; and headaches with a history of systemic disease.6) In clinical interview, the question “Have you experienced the same headache before?” is very useful. If the headache has never been experienced before or is the worst headache ever experienced in life, then it is important to conduct neurological examinations and evaluations, and select appropriate imaging studies, blood tests and cerebrospinal fluid test.7) Start treatment if the test and examination results exclude secondary headaches with high emergency, such as subarachnoid hemorrhage, and do not contradict with a diagnosis of primary headache. If the clinical course is not typical of primary headache or if response to treatment is poor, reconsider the possibility of secondary headache.8) Especially, in a patient with primary headache who becomes affected by a disease that causes secondary headache, careful examination is needed so as not to delay the diagnosis.
Secondary headache has to be suspected and imaging studies are required in children with headaches that do not respond to drugs within 6 months; headaches associated with papilloedema, nystagmus, or gait/motor disorder; headaches with no family history of migraine; headaches associated with impaired consciousness or nausea; recurring headaches during sleep causing wakening; and headaches with a family history or medical history of central nervous system disease.9)
Although history taking and physical/neurological examinations are important for the differentiation between primary and secondary headaches, the significance of diagnostic imaging has also been pointed out.10) According to the study of Mayer et al.,11) 54 of 217 patients (25%) who had subarachnoid hemorrhage were misdiagnosed. The misdiagnoses included meningitis (15%), migraine (13%), headache of unknown etiology (13%), cerebral infarction (9%), headache attributed to arterial hypertension (7%), and tension-type headache (7%). Cautions in the diagnosis of subarachnoid hemorrhage are described in a separate CQ (CQ 1-3, page 8), and will not be discussed here.
• References
1) Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33(9): 629-808.
2) The Headache Classification Committee of the International Headache Society (author), International Headache Classification Promotion Committee of Japanese Headache Society (translator): Japanese Edition of the International Classification of Headache Disorders 3rd edition (beta version). Igakushoin, 2014. (In Japanese)
3) Olesen J, Steiner T, Bousser MG, Diener HC, Dodick D, First MB, Goadsby PJ, Gobel H, Lainez MJ, Lipton RB, Nappi G, Sakai F, Schoenen J, Silberstein SD: Proposals for new standardized general diagnostic criteria for the secondary headaches. Cephalalgia 2009; 29(12): 1331-1336.
4) Takeshima T, Manaka S, Igarashi H, Hirata K, Yamane K, Sakai F: International Headache Classification Promotion Committee of Japanese Headache Society: On the proposed revision of the diagnostic criteria for secondary headaches in the International Classification of Headache Disorders 2nd Edition (ICHD-II). Japanese Journal of Headache 2010; 36(3): 235-238. (In Japanese)
5) Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24(Suppl 1): 9-160.
6) Evans RW: Diagnostic testing for migraine and other primary headaches. Neurol Clin 2009; 27(2): 393-415.
7) Takeshima T, Kanki R, Yamashita S: The know-how to diagnose secondary headaches. Chiryo 2011; 93(7): 1544-1549. (In Japanese)
8) Silberstein SD, Lipton RB, Dalessio DJ: Overview, Diagnosis and Classification of headache. Silberstein SD, Lipton RB, Dalessio DJ (eds): Wolff’s Headache and other Head Pain 7th ed, pp6-26, Oxford University Press, Oxford, 2001.
9) Medina LS, D’Souza B, Vasconcellos E: Adults and children with headache: evidence-based diagnostic evaluation. Neuroimaging Clin N Am 2003; 13(2): 225-235.
10) Aygun D, Bildik F: Clinical warning criteria in evaluation by computed tomography the secondary neurological headaches in adults. Eur J Neurol 2003; 10(4): 437-442.
11) Mayer PL, Awad IA, Todor R, Harbaugh K, Varnavas G, Lansen TA, Dickey P, Harbaugh R, Hopkins LN: Misdiagnosis of symptomatic cerebral aneurysm. Prevalence and correlation with outcome at four institutions. Stroke 1996; 27(9): 1558-1563.
• Search terms and secondary sources
• Search database: Pub Med (2012/4/30)
{secondary headache} & {diagnosis} 2351
• Search database: Ichushi for articles published in Japanese (2012/4/30)
{secondary headache} & {diagnosis}212
• One reference added by manual search (reference 7)
CQ I-3
How is subarachnoid hemorrhage diagnosed?
Recommendation
• When subarachnoid hemorrhage is suspected, a rapid and precise diagnosis and treatment by specialist are necessary.
• The typical symptom is “sudden excruciating headache never experienced before”.
• Subarachnoid hemorrhage may manifest warning symptoms from mild bleeding. Pay attention when there is abrupt onset of headache accompanied by nausea or vomiting, dizziness, diplopia or impaired vision, and delirium.
• Regarding neuroimaging, early-stage CT or fluid-attenuated inversion recovery (FLAIR) MR imaging has high diagnostic value.
• When subarachnoid hemorrhage is strongly suspected, a lumbar puncture should be considered even when neuroimaging is negative.
• Several days following the onset of headache, cerebral ischemic symptoms may appear due to cerebral vasoconstriction.
| Grade A |
Background and Objective
Subarachnoid hemorrhage caused by a ruptured cerebral aneurysm has poor outcome. Since misdiagnosis or delay in diagnosis may worsen the outcome, the objective of this section is to improve the capability of the primary care physician to differentiate subarachnoid hemorrhage from other conditions.
In this section, the diagnostic criteria in the International Classification of Headache Disorders 3rd Edition beta version (ICHD-3beta) are provided, and updated knowledge is added.
Comments and Evidence
Guidelines for the diagnosis and treatment of subarachnoid hemorrhage have been published in Japan and overseas.1)2) The prognosis of subarachnoid hemorrhage is poor; overall mortality of 25-53% has been reported.3)4) The most important factor that aggravates the prognosis is rebleeding from the ruptured cerebral aneurysm. Since rebleeding is a common cause of misdiagnosis and delay in diagnosis, an accurate diagnosis together with treatment provided by specialist are essential.5)6) Before the onset of the major attack of subarachnoid hemorrhage accompanied by “abrupt onset of the worst headache ever experienced”,3) minor leak occurs in around 20% of the patients. Misdiagnosis of these warning leaks would deteriorate the outcome; therefore attention has to be given to these cases.7)8) The most common symptom of minor leak is sudden headache, but may be accompanied by nausea or vomiting, dizziness, delirium,9) oculomotor paralysis, and visual disturbance.10) Careful history taking is essential. The common neck stiffness is not observed during the very early stage of subarachnoid hemorrhage, therefore be aware that “absence of neck stiffness does not exclude a diagnosis of subarachnoid hemorrhage”. CT is a useful neuroimaging modality. The diagnostic power increases by comparing with former images.11)12) The diagnostic rate is 98-100% when performed within 12 hours of onset.13)-15) When a CT scan shows no abnormality, FLAIR MR imaging is useful.8)16)-18) Even when imaging findings are negative, a lumbar puncture for cerebrospinal fluid examination is important, especially at 12 hours or later after onset.1)2)4)13)19)
• For Reference
According to the International Classification of Headache Disorders 3rd Edition beta version (ICHD-3beta) published in 2013, the diagnostic criteria for 6.2.2 Headache attributed to non-traumatic subarachnoid hemorrhage are as follows16):
A. Any new headache fulfilling criterion C
B. Subarachnoid haemorrhage (SAH) in the absence of head trauma has been diagnosed
C. Evidence of causation demonstrated by at least two of the following:
1. headache has developed in close temporal relation to other symptoms and/or clinical signs of SAH, or has led to the diagnosis of SAH
2. headache has significantly improved in parallel with stabilization or improvement of other symptoms or clinical or radiological signs of SAH
3. headache has sudden or thunderclap onset
D. Not better accounted for by another ICHD-3 diagnosis.
• References
1) Yoshimine T (Ed.): Evidence-based guidelines for the management of aneurysmal subarachnoid hemorrhage, the second edition. Surg Cereb Stroke 2008; 36(Suppl I): 1-80. (In Japanese)
2) Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE Jr, Harbaugh RE, Patel AB, Rosenwasser RH; American Heart Association: Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 2009; 40(3): 994-1025.
3) Talavera JO, Wacher NH, Laredo F, Halabe J, Rosales V, Madrazo I, Lifshitz A: Predictive value of signs and symptoms in the diagnosis of subarachnoid hemorrhage among stroke patients. Arch Med Res 1996; 27(3): 353-357.
4) van Gijn J, Kerr RS, Rinkel GJ: Subarachnoid haemorrhage. Lancet 2007; 369(9558): 306-318.
5) Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL: The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg 1990; 73(1): 18-36.
6) Inagawa T: Delayed diagnosis of aneurysmal subarachnoid hemorrhage in patients: a community-based study. J Neurosurg 2011; 115(4): 707-714.
7) Bassi P, Bandera R, Loiero M, Tognoni G, Mangoni A: Warning signs in subarachnoid hemorrhage: a cooperative study. Acta Neurol Scand 1991; 84(4): 277-281.
8) Jakobsson KE, Saveland H, Hillman J, Edner G, Zygmunt S, Brandt L, Pellettieri L: Warning leak and management outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg 1996; 85(6): 995-999.
9) Caeiro L, Menger C, Ferro JM, Albuquerque R, Figueira ML: Delirium in acute subarachnoid haemorrhage. Cerebrovasc Dis 2005; 19(1): 31-38.
10) McCarron MO, Alberts MJ, McCarron P: A systematic review of Terson’s syndrome: frequency and prognosis after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2004; 75(3): 491-493.
11) Johansson I, Bolander HG, Kourtopoulos H: CT showing early ventricular dilatation after subarachnoidal hemorrhage. Acta Radiol 1992; 33(4): 333-337.
12) van der Wee N, Rinkel GJ, Hasan D, van Gijn J: Detection of subarachnoid haemorrhage on early CT: is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry 1995; 58(3): 357-359.
13) Liebenberg WA, Worth R, Firth GB, Olney J, Norris JS: Aneurysmal subarachnoid haemorrhage: guidance in making the correct diagnosis. Postgrad Med J 2005; 81(957): 470-473.
14) Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd: Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. Am J Neuroradiol 2003; 24(2): 254-256.
15) Boesiger BM, Shiber JR: Subarachnoid hemorrhage diagnosis by computed tomography and lumbar puncture: are fifth generation CT scanners better at identifying subarachnoid hemorrhage? J Emerg Med 2005; 29(1): 23-27.
16) The Headache Classification Committee of International Headache Society: International Classification of Headache Disorders 3rd Edition beta version 2013. Cephalalgia 33 (9): 696-697.
17) Leblanc R: The minor leak preceding subarachnoid hemorrhage. J Neurosurg 1987; 66(1): 35-39.
18) Mitchell P, Wilkinson ID, Hoggard N, Paley MN, Jellinek DA, Powell T, Romanowski C, Hodgson T, Griffiths PD: Detection of subarachnoid haemorrhage with magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2001; 70(2): 205-211.
19) Wood MJ, Dimeski G, Nowitzke AM: CSF spectrophotometry in the diagnosis and exclusion of spontaneous subarachnoid haemorrhage. J Clin Neurosci 2005; 12(2): 142-146.
• Search terms and secondary sources
• Search database: PubMed (2011/10/15)
Subarachnoid hemorrhage diagnosis
& human
& English/Japanese
& 2005-
& practical guideline/review = 457 articles
Cerebral aneurysm
& Subarachnoid hemorrhage diagnosis
& human & English/Japanese & 2005-
& RCT/metaanalysis = 51 articles
CQ I-4
What are the procedures for managing headache in the emergency room?
Recommendation
For patients presenting with a major complaint of headache, differentiation between primary headache and secondary headache is the most important. First screening for life-threatening headaches should be performed, with special attention to headache due to subarachnoid hemorrhage. History taking, physical and neurological examination, and neuroimaging (CT/MRI) are important for a diagnosis of headache. Even when neuroimaging shows no abnormality, lumbar puncture should be considered if subarachnoid hemorrhage is strongly suspected.
| Grade A |
Background and Objective
Patients with diverse complaints of headaches visit the emergency room, ranging from highly emergent subarachnoid hemorrhage to primary headaches. According to the data (between January 1997 and December 1999) of the emergency outpatient department of Keio University Hospital, headache emergencies occupied 3.2% of all emergency cases, 38.3% of which were primary headaches (including migraine 6.6%) and 53.6% were secondary headaches, with subarachnoid haemorrhage constituting 8.1%.1) In an emergency department of a hospital in the United States, the vast majority of patients who presented with acute primary headache had migraine (95%).2) However, the emergency department physicians diagnosed migraine in only 32% of the patients, and only 7% of the patients received medications specific for migraine. Emergency physicians are required to have the competency to diagnose secondary headaches, and the knowledge to diagnose and treat primary headaches.
Comment and Evidence
First, physicians should know about headache classification as described in the International Classification of Headache Disorders 2nd Edition (ICHD-II).3)4) A sinister headache should be suspected if the onset and clinical course fulfill the following criteria5): patient is younger than 5 years or older than 50 years; new onset headache within the past 6 months; very acute course reaching the highest intensity within 5 minutes; atypical symptoms, headache accompanied by symptoms never before experienced; presence of local neurological abnormalities; non-resolving neurological symptoms; presence of rash, head tenderness, head injury, infection, and hypertension.
Dodick6) proposed concise and easy to understand clinical clues for the differentiation between primary and secondary headaches, abbreviated as SNOOP.
SNOOP: Clinical clues for clinical diagnosis
Systemic symptoms/signs (fever, myalgias, weight loss)
Systemic disease (malignancy, acquired immune deficiency syndrome)
Neurologic symptoms or signs
Onset sudden (thunderclap headache)
Onset after age 40 years
Pattern change (progressive headache with loss of headache-free periods, change in type of headache)
In a study connected on 264 patients visiting an internal medicine department with a complaint of headache but no neurological abnormalities, patients were asked three questions: Q1 “Is your headache the worst ever? (worst)”, Q2 “Is your headache getting worse? (worsening)”, and Q3 “Was the onset of headache sudden? (sudden)”.7) Among the three questions, Q2 (worsening) had the highest positive predictive value, followed by Q1 (worst). It is noteworthy that none of the patients who were negative for all three questions had red flag headaches.
Cortelli et al.8) proposed evidence-based diagnosis of non-traumatic headache in the emergency department (ER). They summarized the consensus regarding four clinical scenarios based on extensive literature review.
Scenarios for the diagnosis of non-traumatic acute headache
• Scenario 1
Adult patients admitted to ER for severe headache (“worst headache”)
* with acute onset ( “thunderclap headache”)
* with focal neurological findings (or non-focal, such as decreased level of consciousness)
* with vomiting or syncope at onset of headache
→ Perform head CT
→ If CT scan is negative or uncertain, or of poor quality, perform lumbar puncture
→ If lumbar puncture shows no abnormality, evaluation by a neurologist within 24 hours is necessary
•Scenario 2
Adult patients admitted to ER for severe headache
* With fever and/or neck stiffness
→ Perform head CT and lumbar puncture
• Scenario 3
Adult patients admitted to ER for the following conditions:
* headache of recent onset (days or weeks)
* progressively worsening headache, or persistent headache
→ Perform head CT
→ Perform routine blood tests (including erythrocyte sedimentation rate and C-reactive protein)
→ If tests are negative, perform neurological evaluation within 7 days
• Scenario 4
Adults with a past history of headache
* Headache similar to previous headache in intensity, duration and associated symptoms
→ Perform vital signs examination, neurological evaluation and routine blood tests
→ If tests are negative, discharge patient from ER
→ After discharge, provide collaborated care
Although the medical care environment in Japan differs in some aspect from other countries, the above diagnostic scenarios provide useful references. When MRI is used as the first neuroimaging method for acute headache, FLAIR or T2-weighted imaging is essential.
Kowalski et al.9) conducted a cohort study on 482 patients with subarachnoid hemorrhage admitted to a tertiary hospital, to analyze the association of initial misdiagnosis with outcome. According to their study, 12% of the patients with subarachnoid hemorrhage were misdiagnosed, and migraine or tension-type headache (36%) was the most common incorrect diagnosis. Misdiagnosis was common in patients with mild bleeding or normal mental status. Misdiagnosis was associated with poor survival and functional outcome. More aggressive CT scanning in patients suspected of subarachnoid haemorrhage, even though the symptoms are mild, may reduce the frequency of misdiagnosis. Even when CT and cerebrospinal fluid test are negative, conducting FLAIR MRI may lead to a diagnosis of subarachnoid haemorrhage.10)
Lewis and Qureshi11) analyzed the cause of acute headache in children and adolescents (boys and girls). Their results showed that upper respiratory tract infection with fever, sinusitis, and migraine were the most common causes. Physicians have to pay special attention if the acute headache is located in the occipital region or if the patient is unable to describe the quality of the pain. Serious underlying diseases such as brain tumor and intracranial hemorrhage are rare; when present, they are accompanied by multiple neurological signs (such as ataxia, hemiparesis, and papilledema).
• References
1) Yokoyama M, Hori S, Aoki K, Fujishima S, Kimura Y, Suzuki M, Aikawa N: Headache in patients transported to emergency department. Japanese Journal of Headache 2001; 28(1): 4-5. (In Japanese)
2) Blumenthal HJ, Weisz MA, Kelly KM, Mayer RL, Blonsky J: Treatment of primary headache in the emergency department. Headache 2003; 44(10): 1026-1031.
3) Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24(Suppl 1): 9-160.
4) The Headache Classification Committee of International Headache Society: International Classification of Headache Disorders 2nd Edition (ICHD-II). Japanese Journal of Headache 2004; 31(1): 13-188. (In Japanese)
5) Dowson AJ, Sender J, Lipscombe S, Cady RK, Tepper SJ, Smith R, Smith TR, Taylor FR, Boudreau GP, van Duijn NP, Poole AC, Baos V, Wober C: Establishing principles for migraine management in primary care. Int J Clin Pract 2003; 57(6): 493-507.
6) Dodick DW: Clinical clues (primary/secondary), The 14th Migraine Trust International Symposium. London, 2002.
7) Basugi M, Ikusaka M, Mikasa G, Kim S: Usefulness of three simple questions to detect red flag headaches in outpatient settings. Japanese Journal of Headache 2006; 33(1): 30-33.
8) Cortelli P, Cevoli S, Nonino F, Baronciani D, Magrini N, Re G, De Berti G, Manzoni GC, Querzani P, Vandelli A; Multi-disciplinary Group for Nontraumatic headache in the Emergency Department: Evidence-based diagnosis of nontraumatic headache in the emergency department: a consensus statement on four clinical scenarios. Headache 2004; 44(6): 587-595.
9) Kowalski RG, Claassen J, Kreiter KT, Bates JE, Ostapkovich ND, Connolly ES, Mayer SA: Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA 2004; 291(7): 866-869.
10) Ogami R, Igawa F, Ohbayashi N, Imada Y, Hidaka T, Inagawa T: A case of subacute subarachnoid hemorrhage not diagnosed by cerebrospinal fluid examination: usefulness of emergent MRI. Neurological Surgery 2003; 31(6): 663-668. (In Japanese)
11) Lewis DW, Qureshi F: Acute headache in children and adolescents presenting to the emergency department. Headache 2000; 40(3): 200-203.
• Search terms and secondary sources
• Search database: PubMed (2012/5/5)
No. Request & Records
1 Headache 55659
2 emergency 210382
3 #1 & #2 1907
4 etiology 6577149
5 management 1654390
6 diagnosis 7723671
7 therapy 6548922
8 treatment 7421136
9 “differential diagnosis” 391173
10 #3 & #4 1076
11 #3 & #5 704
12 #3 & #6 1405
14 #3 & #8 1324
15 #3 & #9 289
16 #10 or #11 or #12 or #13 or #14 or #15 1829
17 “Evidence-Based-Medicine”/all subheadings 49397
18 guidelines 219788
19 consensus 96334
20 #16 & #17 11824
21 #16 & #18 8
22 #16 & #19 2 23 #20 or #21 or #22 11
• Search database: Ichushi for articles published in Japan (2012/5/5)
(headache) & (emergency) 1103
CQ I-5
How should primary care physicians manage headache?
Recommendation
Primary care physicians should bear in mind to differentiate between primary headaches and secondary headaches, and in case of difficulties with diagnosis, should promptly refer the patient to a specialist. For primary headaches, primary care physicians should be able to correctly diagnose and treat especially migraine and tension-type headache.
| Grade A |
Background and Objective
Headache is one of the common complaints encountered in routine clinical care. It is estimated that primary care physicians accurately diagnose headache at a rate of approximately 50%. The issue for primary care physicians is how to improve the precision of diagnosis and treatment of headache. When providing headache care, primary care physicians should first of all diagnose the cause of headache accurately. To do this requires knowledge regarding the classification of headaches. When primary care physicians with no access to head CT and MRI encounter difficulties in differentiating secondary headaches from primary headaches, they should refer the patient to a specialist as soon as possible. Especially in the case of sudden onset of headache in which subarachnoid hemorrhage cannot be excluded, the patient should be referred to a neurosurgeon.
Although primary headaches are considered not to cause residual organic damage to the brain, headache attacks cause disability in daily life. Therefore, appropriate treatment is required to improve the daily life of the patients.
For clinical care of headache, use simple screeners and headache diary for diagnosis, severity evaluation, and treatment; evaluate the treatment effect appropriately; and it is also important to give proper guidance to the patients about the timing of taking acute medications for headache and on prophylactic treatment.
Comments and Evidence
First, primary care physicians should know about the International Classification of Headache Disorders 2nd Edition (ICHD-II) developed by the International Headache Society (IHS),1) which set out diagnostic criteria for each of the headache types. Furthermore, they should know that according to ICHD-II, headaches are classified into primary headaches and secondary headaches, and that primary headaches include migraine, tension-type headache, and cluster headache, while secondary headaches are caused by various neurological disorders and may include systemic diseases.1) When primary care physicians provide care for headache, it is important that first of all they have knowledge of the diagnostic criteria for primary headaches. Although ICHD-II classifies in a hierarchical manner, primary care physicians should be familiar with at least the first level (for example, the level to diagnose “migraine”). To diagnose primary headaches, it is necessary to exclude the possibility of secondary headaches. In practice, precise history taking, neurological evaluation, sometimes blood tests and neuroimaging are necessary to exclude secondary headaches. If eye disease or disease of other discipline is suspected from the beginning, refer the patient to the respective specialist as soon as possible. When a diagnosis of primary headache is established, plan treatment according to this guideline.
Simple screeners headache for use by primary care physicians have been developed, and reported to have high specificity for the diagnosis of migraine.2)3) One of them consists of questions on the frequency of headache, and the use of medications.2) Another screener contains questions based on the diagnostic criteria of ICHD-II, including the frequency and duration of headache, aura, and degree of disability.3) MIDAS and HIT-6 are tools that evaluate objectively the impact of headache on patient’s activities of daily living. Use these screeners to aid diagnosis and evaluation of severity, and provide treatment appropriate to individual patients. Use headache diary for follow-up observation. Advise patients on the timing of taking medications for migraine. Provide rescue treatment when the early treatment fails. Offer prophylactic treatment when headache occurs frequently. As such, primary care physicians also have to be engaged in many aspects of headache management.4)5)
• References
1) The Headache Classification Committee of the International Headache Society (author), International Headache Classification Promotion Committee of Japanese Headache Society (translator): Japanese Edition of the International Classification of Headache Disorders 2nd Edition. Igakushoin, 2007. (In Japanese)
2) Maizels M, Burchette R: Rapid and sensitive paradigm for screening patients with headache in primary care settings. Headache 2003; 43(5): 441-450.
3) Cousins G, Hijazze S, Van de Laar FA, Fahey T: Diagnostic accuracy of the ID Migraine: a systematic review and meta-analysis. Headache 2011; 51(7): 1140-1148.
4) Dowson AJ, Sender J, Lipscombe S, Cady RK, Tepper SJ, Smith R, Smith TR, Taylor FR, Boudreau GP, van Duijin NP, Poole AC, Baos V, Wober C: Establishing principles for migraine management in primary care. Int J Clin Pract 2003; 57(6): 493-507.
5) Dowson AJ, Lipscombe S, Sender J, Rees T, Watson D; MIPCA Migraine Guidelines Development Group: Migraine in primary care: New guidelines for the management of migraine in primary care. Curr Med Res Opin 2002; 18(7): 414-439.
• Search terms and secondary sources
• Search database: PubMed (2011/12/21)
Headache & ‘primary care’ 1078
Headache & ‘primary care’ & diagnosis 710
Headache & general practitioner 326
Headache & general practitioner & diagnosis 190
Headache & algorithms 171
Headache & screener 15
CQ I-6
How should dentists manage headache?
Recommendation
• Dentists should differentiate between headache and temporomandibular disorder.
• In the differential diagnosis of toothache of unknown cause, the possibility of the involvement of the teeth by primary headaches and secondary headaches has to be considered.
• Cases with concurrent headache which are difficult to diagnose should be referred promptly to specialists.
| Grade B |
Background and Objective
Temporomandibular disorder occurs overwhelmingly more often in women, and is known to be a disease with gender difference. Primary headaches, especially migraine and tension-type headache, tend to occur concurrently with temporomandibular disorder. Moreover, since the pain experienced by patients with cluster headache and migraine sometimes involves the face and the teeth, these patients may visit dentists with the major complaint of toothache or temporomandibular pain. Dentists are recommended to have the capability of differentiating these headaches from temporomandibular disorder and odontogenic pain.
On the other hand, it has been reported that dental disease may be a cause of secondary headaches.
Comments and Evidence
In the International Classification of Headache Disorders 3rd Edition (beta version) (ICHD-3beta) of the International Headache Society (IHS),1) tension-type headache is subdivided into infrequent episodic tension-type headache, frequent episodic tension-type headache, and chronic tension-type headache; and each further subdivided into two subforms: with and without pericranial tenderness. Increased pericranial tenderness induced by palpation is the most significant abnormal finding in patients with tension-type headache. The tenderness increases with the intensity and frequency of headache, and is further increased during actual headache. Pericranial tenderness is in fact tenderness of the frontal muscle, temporal muscle, masseter muscle, lateral and medial pterygoid muscle, sternocleidomastoid muscle, splenius muscle, and trapezius muscle. In another words, tension-type headache and myogenic temporomandibular disorder may be regarded as similar diseases with the same source of pain but different pain reception sites. Because the muscles are affected, stiff shoulders and stiff neck often occur concurently.2)3)
In addition, studies have shown a pathological association between temporomandibular disorder and headache, and between toothache and headache.4)5)
Migraine is a disease with high prevalence, and therefore may coexist incidentally with other diseases that have high prevalence. A report has indicated that one-half of the patients with temporomandibular disorder have migraine concurrently. Patients with migraine sometimes manifest allodynia in the crainocervical region both during headache and when in remission, probably a result of lowered threshold of pericranial tenderness.6) Furthermore, the pain in migraine not only involves the first division of the trigeminal nerve, but also the second and third divisions, and may sometimes be misdiagnosed as temporomandibular disorder or toothache.7) This is a result of sensitization of the central nervous system due to headache attack, and conversely deep pain in the craniocervical region may also sensitize the central nervous system. Consequently, temporomandibular disorder is a factor that contributes to aggravate headache frequency or induce chronicity of headache.4)5)
• References
1) Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33(9): 629-808.
2) Jensen R, Rasmussen BK, Pedersen B, Olesen J: Muscle tenderness and pressure pain thresholds in headache. A population study. Pain 1993; 52(2): 193-199.
3) Schmidt-Hansen PT, Svensson P, Bendtsen L, Graven-Nielsen T, Bach FW: Increased muscle pain sensitivity in patients with tension-type headache. Pain 2007 ; 129(1-2): 113-121.
4) Bevilaqua-Grossi D, Lipton R, Bigal ME: Temporomandibular disorders and migraine chronification. Curr Pain Headache Rep 2009; 13(41): 314-318.
5) Goncalves DA, Speciali JG, Jales LC, Camparis CM, Bigal ME: Temporomandibular symptoms, migraine, and chronic daily headaches in the population. Neurology 2009; 73(8): 645-646.
6) Bevilaqua-Grossi D, Lipton RB, Napchan U, Grosberg B, Ashina S, Bigal ME: Temporomandibular disorders and cutaneous allodynia are associated in individuals with migraine. Cephalalgia 2010; 30(4): 425-432.
7) Graff-Radford SB: Headache problems that can present as toothache. Dent Clin North Am 1991; 35(1): 155-170.
• Search terms and secondary sources
• Search database: PubMed (2011/12/21)
headache & dental pain 537
TMD & migraine headache 33
TMD & tension-type headache 38
CQ I-7
Are headache clinic and headache specialist necessary? Is collaborative care useful for primary headaches?
Recommendation
Headache clinic is necessary to improve the satisfaction and quality of life (QOL) of patients with chronic headache. In the headache clinic, diagnosis and treatment should be provided by headache specialists with expert knowledge not only in highly emergent secondary headaches but also in chronic headaches. Especially, when primary care physicians have difficulties with diagnosis or treatment of headache, referral to or consultation with headache specialists is recommended. Collaboration between primary care physicians and headache specialists for the management of primary headaches increases the satisfaction and QOL of patients. Collaborative care for primary headaches should be further promoted.
| Grade A |
Background and Objective
Many patients with chronic headaches have headaches that seriously interfere with their daily activities. Yet, the needs of the patients were not met. Many patients either never sought medical care or were not diagnosed and treated appropriately even if they had received medical care, while others were always anxious that as the doses of analgesics increased, the medications might become ineffective. To address this situation, the Japanese Headache Society started to certify headache specialists from 2005, and began to establish headache clinics nationwide. A nationwide epidemiological survey in Japan estimated that approximately 40 million persons were affected by chronic headache.1) The numbers of headache specialists and headache clinics remain insufficient.
Comment and Evidence
According to a nationwide epidemiological survey in Japan, the number of persons affected by headache was estimated to be approximately 40 million, 8.5 million of whom had migraine and 74% of whom had serious disability in daily living because of the headache.1) The economic loss because of headache, including direct loss due to medical expenses and indirect loss due to the incapability to work, amounts to nearly three hundred billion yen a year.2) The World Health Organization (WHO) ranked migraine at the 19th place among diseases that shorten the healthy lifespan.3) Approximately 70% of migraine patients never consult medical facilities, and approximately 50% are taking only over-the-counter medications.1)4) Most of the patients with chronic headache who have never consulted a medical facility, patients who have not been appropriately diagnosed, and patients who are treated only with over-the-counter medications have serious disability in daily living. In addition, even among those who have consulted medical facilities, many are not accurately diagnosed and do not receive appropriate treatment.5)6) In the background of such situation, issues on the medical facility side include the following: (1) only neuroimaging is conducted to exclude organic diseases, and the diagnosis for migraine is inadequate; (2) even when migraine is diagnosed, knowledge on treatment is inadequate leading to patient dissatisfaction; and (3) diagnosis and treatment are not explained adequately to patients. On the other hand, there are also issues on the patient’s side, including: (4) feel assured by exclusion of organic diseases alone, and do not ask for treatment; and (5) are embarrassed by consulting medical facilities because of headache, due to a lack of understanding that migraine is a condition that requires treatment.7) Through the establishment and publicity of headache clinics, the number of patients with chronic headache consulting headache specialists has increased.5)-9) When the headache clinic was opened at the Department of Neurology at Yamaguchi University, the event was publicized in the press and television, resulting in an increase of new headache patients by 7.4-fold, especially with a significant increase in patients with migraine.8) Among patients with migraine consulting the headache clinic, their primary purpose is to seek treatment, followed by to know the cause of their headache.7) In a study of 38 patients with migraine referred by primary care physicians to a specialist headache clinic in Singapore, the pain intensity, MIDAS score, and SF-36 score improved after three months, and patient satisfaction also increased.10) Referral from general physicians to headache specialists benefits the patients by ameliorating the fear toward headache, improving the headache per se, and improving QOL.11)12)
To improve headache care, experienced headache specialists and headache clinics staffed by headache specialists are essential.9) An accurate diagnosis of headache and every possible approach to relieve the disease burden of headache patients should be provided.
• References
1) Sakai F, Igarashi H: Prevalence of migraine in Japan: a nationwide survey. Cephalalgia 1997; 17(1): 15-22.
2) Sakai F: [Special Issue: Primary Care for Headache] Headache diagnosis system (headache specialist, headache clinic, medical collaboration). Chiryo 2011; 93(7): 1609-1613. (In Japanese)
3) World Health Organization: The World Health Report 2001-Mental Health: New Understanding, New Hope. http: //www.who.int/whr/2001/en/
4) Takeshima T, Ishizaki K, Fukuhara Y, Ijiri T, Kusumi M, Wakutani Y, Mori M, Kawashima M, Kowa H, Adachi Y, Urakami K, Nakashima K: Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache 2004; 44(1): 8-19.
5) Lipton RB, Stewart WF, Simon D: Medical consultation for migraine: results from the American Migraine Study. Headache 1998; 38(2): 87-96.
6) Lipton RB, Scher AI, Steiner TJ, Bigal ME, Kolodner K, Liberman JN, Stewart WF: Patterns of health care utilization for migraine in England and in the United States. Neurology 2003; 60(3):441-448.
7) Tada Y, Negoro Y, Ogasawara J, Kawai M, Morimatsu M: A dramatic increase in the number of outpatients with migraine after the opening of a headache clinic. Yamaguchi Igaku 2003; 52(5): 169-173. (In Japanese)
8) Kakinuma S, Negoro K, Tada Y, Morimatsu H: Effects of mass media announcements on the number of outpatients visiting a headache clinic. Shinkei Iryo 2003; 20(1): 63-69. (In Japanese)
9) Sakai F (Ed.) Negoro Y, Tada Y: Headache clinic. Latest Medicine Supplement [ABC of New Diagnosis and Treatment 21/ Neurology 2 Headache 2004: 26-32. (In Japanese)
10) Soon YY, Siow HC, Tan CY: Assessment of migraineurs referred to a specialist headache clinic in Singapore: diagnosis, treatment strategies, outcomes, knowledge of migraine treatments and satisfaction. Cephalalgia 2005; 25(12): 1122-1132.
11) Bekkelund SI, Salvesen R; North Norway Headache Study (NNHS): Are headache patients who initiate their referral to a neurologist satisfied with the consultation? A population study of 927 patients — the North Norway Headache Study (NNHS). Fam Pract 2001; 18(5): 524-527.
12) Salvesen R, Bekkelund SI: Aspects of referral care for headache associated with improvement. Headache 2003; 43(7): 779-783.
• Search terms and secondary sources
• Search database: PubMed (2012/4/30)
{headache clinic} 3175
& ({role} OR {necessity}) 232
&specialist 62
(1) & {medical treatment} & {migraine} 73
• Search database: Ichushi Web for articles published in Japan (2012/4/30)
headache clinic 142
specialist headache clinic 12
headache center 28
headache specialist 7
• Secondary source: 4 references from manual search (references 1, 3, 4 and10)
CQ I-8
How are algorithms used?
Recommendation
The diagnosis and treatment of headache start from differentiating secondary headaches, especially the dangerous (life-threatening) headaches. Next, the primary headaches, including migraine, should be diagnosed. Simple diagnostic algorithms are a powerful tool that provides clues to the diagnosis of headaches in the clinical setting.
| Grade B |
Background and Objective
The objective of this section is to illustrate how algorithms can be used for effective diagnosis of headache in the busy routine clinical setting.
Comments and Evidence
The diagnosis and treatment of headache start from excluding the secondary headaches that are dangerous headaches. An algorithm for use by primary care physicians is available (Figure 1). After screening for dangerous headaches, the diagnosis of chronic headaches that are primary headaches including migraine then begins.1)-4) The algorithm comprises four major questions: “What is the impact of the headache on daily life?”, “How many days of headache in a month?”, “how many days per week are medications taken?” and “Does the attack start with reversible homonymous visual symptoms or unilateral sensory symptoms?”2) (Figure 2).
For migraines, “POUNDing” that is composed of the acronyms characterizing the five symptoms of migraine is useful.4) POUNDing stands for Pulsating, duration of 4-72 hOurs, Unilateral, Nausea, and Disabling. If four of the five are satisfied, then there is a high probability of migraine (Figure 3). Moreover, another algorithm examines the common clinical question of what kinds of patients require neuroimaging. Six items: “cluster-type headache”, “abnormal findings on neurologic examination”, “undefined headache (not cluster-, migraine-, or tension-type)”, “headache with aura”, “headache aggravated by exertion or valsalva-like maneuver”, and “headache with vomiting”, are useful in judging whether neuroimaging is necessary (Figure 4). An algorithm for differentiating chronic daily headaches5) and another algorithm for the management of primary headaches in the emergency setting6) have also been reported.
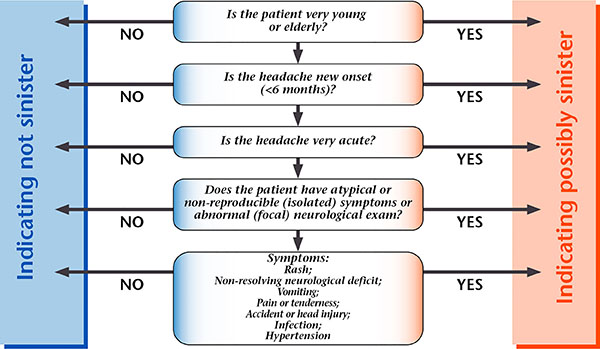
Sinister Headache Algorithm @2004 www.pico.org.uk
Figure 1. Simple diagnostic algorithm for screening sinister headache.
Reproduced with permission from Migraine Action.
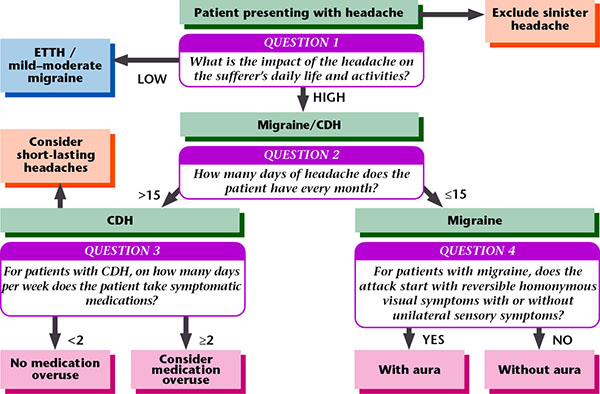
GP Diagnostic Algorithm @2004 www.pico.org.uk
Figure 2. Algorithm for screening headache.
Reproduced with permission from Migraine Action.
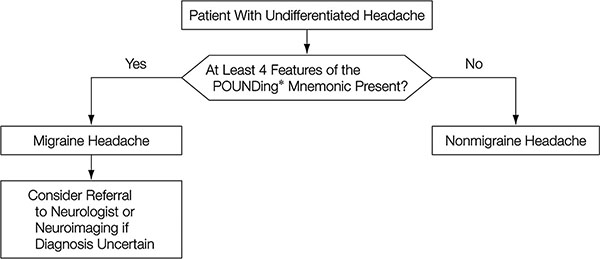
Figure 3. Algorithm for the approach to headache: Does this patient have a migraine headache?
*POUNDing: Pulsatile; duration 4-72 hOurs; Unilateral; Nausea; Disabling
[Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM: Does this patient with headache have a migraine or need neuroimaging? JAMA 2006;296(10):1274-1283. Copyright © (2006) American Medical Association. All rights reserved.]
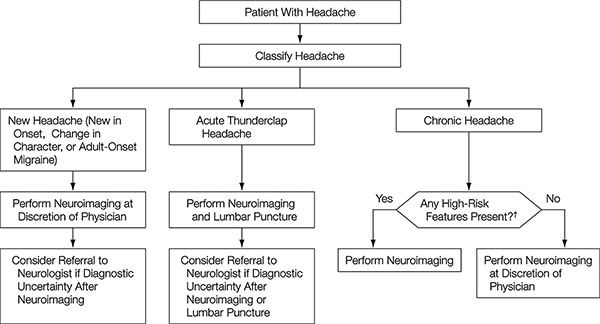
Figure 4. Algorithm for the approach to headache: Does this patient need neuroimaging?
†Cluster-type headache, abnormal findings on neurologic examination, undefined headache (not cluster-, migraine-, or tension-type), headache with aura, headache aggravated by exertion or valsalva-like maneuver, headache with vomiting
[Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM: Does this patient with headache have a migraine or need neuroimaging? JAMA 2006;296(10):1274-1283. Copyright © (2006) American Medical Association. All rights reserved.]
• References
1) Dowson AJ, Sender J, Lipscombe S, Cady RK, Tepper SJ, Smith R, Smith TR, Taylor FR, Boudreau GP, van Duijn NP, Poole AC, Baos V, Wöber C: Establishing principles for migraine management in primary care. Int J Clin Pract 2003; 57(6): 493-507.
2) Dowson AJ, Bradford S, Lipscombe S, Rees T, Sender J, Watson D, Wells C: Managing chronic headaches in the clinic. Int J Clin Pract 2004; 58(12): 1142-1151.
3) Pryse-Phillips W, Aube M, Gawel M, Nelson R, Purdy A, Wilson K: A headache diagnosis project. Headache 2002; 42(8): 728-737.
4) Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM: Does this patient with headache have a migraine or need neuroimaging? JAMA 2006; 296(10): 1274-1283.
5) Bigal ME, Lipton RB: The differential diagnosis of chronic daily headaches: an algorithm-based approach. J Headache Pain 2007; 8(5): 263-272.
6) Torelli P, Campana V, Cervellin G, Manzoni GC: Management of primary headaches in adult Emergency Departments: a literature review, the Parma ED experience and a therapy flow chart proposal. Neurol Sci 2010; 31(5): 545-553.
• Search terms and secondary sources
• Search database: PubMed (2011/10/18)
headache 54858
& diagnosis 32183
& algorithm 170
• Search database: Ichushi Web for articles published in Japan (2011/10/18)
headache 22226
& diagnosis 12004
& algorithm 21
CQ I-9
How is the impact of headache on individuals measured?
Recommendation
Use of questionnaires that have been validated for reliability and validity is recommended to measure the impact of headache on individuals.
| Grade B |
Background and Objective
Impact has a similar connotation to “disability” as defined by the WHO, which is the limitation or incapability of normal activities as a human being. Rather than the subjective manifestation of signs and symptoms and health-related quality of life (HRQOL), the impact of headache is rated as the objective influence of the disease on life activities such as work and leisure activities. Among the primary headaches, the disability caused by migraine has been reported worldwide. The evaluate the severity of migraine, assessing the impact of migraine is important.
Comments and Evidence
Several scales are available for the evaluation of the disability in daily living caused by chronic headache; however, the scales that can be used in Japanese language are limited. This section comments on several questionnaires, including Japanese versions, for the evaluation of the impact of headache in general, which have been reported to have high reliability and validity.
• Headache Impact Questionnaire (HImQ)
This is a scale developed based on the Chronic Pain Inventory (CPI) for measuring the impact of headache. The scale is a 16-item self-administered questionnaire: number of headaches; headache duration; pain intensity; disability; and time lost in work for pay, housework and non-work activities. The scale can be applied to all headaches and has wide utility. However, scoring is complicated, and is therefore more suitable for research than for primary care.1)
• Migraine Disability Assessment (MIDAS)
This is a brief questionnaire based on a part of HImQ. The MIDAS divides daily living into work or school, household work, and non-work activities. The missed days in work and other activities are scored and the total score is used to evaluate the disability. The scale is useful not only for migraine but also for headache in general.2) The MIDAS has been translated into various languages including Japanese, and the reliability and validity have been evaluated.3)
• Headache Impact Test (HIT)
The HIT is composed of items from several widely used QOL and daily living disability scales with proven validity; the Headache Disability Inventory (HDI), Headache Impact Questionnaire (HIQ), MIDAS, and Migraine-Specific Quality of Life Questionnaire (MSQ), together with added questions from clinicians and QOL specialists. It is a tool for measuring the impact of headache on individuals in their ability to function on the job, at school, at home and in social situations. The scale is in the form of an internet-administered questionnaire (only available in English).4)
• HIT-6
The HIT-6 was developed through the construction of the HIT. The questionnaire can be administered as a short paper-based test consisting of six questions that can be responded within one minute. The questions are on pain intensity, impact on daily activities, impact on social activities, and mental burden due to headache. The respondent chooses from one of five choices for each question. Each choice has a predetermined score, and the total score for all six questions is calculated. Based on the total score, the impact on daily living is classified into four grades.5) A high correlation has been found between the HIT-6 score and HIT score. The scale has been translated into more than 25 languages. The reliability of the Japanese version has also been validated.6)
• Migraine Work and Productivity Loss Questionnaire (MWPLQ)
The impact of headache can be measured by focusing on productivity at work.7)
• Headache Needs Assessment (HANA)
The HANA is a questionnaire consisting of 7 items that evaluate the frequency of loss of QOL and bothersomeness.8)
• References
1) Stewart WF, Lipton RB, Simon D, Von Korff M, Liberman J: Reliability of an illness severity measure for headache in a population sample of migraine sufferers. Cephalalgia 1998; 18(1): 44-51.
2) Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN: Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000; 88(1): 41-52.
3) Iigaya M, Sakai F, Kolodner KB, Lipton RB, Stewart WF: Reliability and validity of the Japanese Migraine Disability Assessment (MIDAS) Questionnaire. Headache 2003; 43(4): 343-352.
4) Bayliss MS, Dewey JE, Dunlap I, Batenhorst AS, Cady R, Diamond ML, Sheftell F: A study of the feasibility of Internet administration of a computerized health survey: the headache impact test (HIT). Qual Life Res 2003; 12(8): 953-961.
5) Kosinski M, Bayliss MS, Bjorner JB, Ware JE Jr, Garber WH, Batenhorst A, Cady R, Dahlof CG, Dowson A, Tepper S: A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 2003; 12(8): 963-974.
6) Sakai F, Fukuuchi Y, Iwada M, Hamada J, Igarashi H, Shimizu T, Suyama K, Kageyama S, Arakawa I, Ijiri T, Uechi Y, Nagata T. Evaluation of reliability of the Japanese version “Headache Impact Test (HIT-6)”. Rinsho Iyaku 2004; 20(10): 1045-1054. (In Japanese)
7) Davies GM, Santanello N, Gerth W, Lerner D, Block GA: Validation of a migraine work and productivity loss questionnaire for use in migraine studies. Cephalalgia 1999; 19(5): 497-502.
8) Cramer JA, Silberstein SD, Winner P: Development and validation of the Headache Needs Assessment (HANA) survey. Headache 2001; 41(4): 402-409.
• Search terms and secondary sources
• Search database: PubMed(2011/8/28)
Headache All fields 54478
& {impact} 1284
& {burden} 94
& {QOL} 32
• Search database: Ichushi Web for articles published in Japan (2011/12/21)
headache 795
& {QOL and/or quality of life}12
& {disability}1
& {burden}0
& {impact}0
CQ I-10
How are questionnaires and screeners used?
Recommendation
Questionnaires on headache include those that measure the disability in daily living, QOL, treatment effect and satisfaction, as well as diagnostic screeners for the diagnosis of migraine. Use of these questionnaires and screeners contributes to routine clinical care by improving the communication between patients and doctors, and providing simple and rapid diagnosis as well as objective evaluation of therapeutic effects.
| Grade B |
Background and Objective
Although a careful medical interview is important for the diagnosis and treatment of headache, it is difficult to obtain sufficient information from patients during the busy consultation hours. Various interview sheets and screeners have been developed to support the routine clinical care for primary headaches, with the objective to attain accurate diagnosis and treatment as well as effective communication between doctors and patients.
Comments and Evidence
The following interview sheets and screeners for headache have been evaluated for reliability and validity.
Diagnostic screeners
(1) 3-Question Headache Screen
(2) ID Migraine
The 3-Question Headache Screen1) diagnoses migraine from three features: (1) recurrent headaches that are disabling (2) headaches lasting at least 4 hours and (3) no new or different headaches in the past 6 months.
The ID Migraine2) diagnoses migraine from three items: disability, nausea and sensitivity to light. Because the screener is simple and can be self-administered, its usefulness in primary care is attracting attention. In Japan also, similar validation study was conducted as a multi-center, blinded, clinical epidemiological study2b).
Questionnaires on disability and severity
(1) Headache Impact Questionnaire (HImQ)
(2) Migraine Work and Productivity Loss Questionnaire (MWPLQ)
(3) Migraine Disability Assessment (MIDAS) Questionnaire
(4) PedMIDAS
(5) Headache Impact Test (HIT)
(6) HIT-6
MIDAS and HIT are examples of short questionnaires.
The MIDAS questionnaire 3)-5) is a short questionnaire developed based on the HImQ. It divides daily living into work or school, household work and non-work activities, and evaluates the degree of disability from the missed days of these activities.3)4) This scale is useful not only for migraine but also for headache in general. It has been translated in various languages including Japanese,5) and the reliability and validity have been evaluated. In addition, MIDAS for adolescents and children, PedMIDAS6) has also been developed and is useful for the evaluation of pediatric headache.
The HIT is composed of items from several widely used QOL and daily living disability scales with proven validity; the Headache Disability Inventory (HDI), Headache Impact Questionnaire (HIQ), MIDAS, and Migraine-Specific Quality of Life Questionnaire (MSQ), together with added questions from clinicians and QOL specialists. It was developed as a tool for measuring the impact of headache on individuals in their ability to function on the job, at home, at school and in social situations. The scale is only available in English. The test is internet-administered, and evaluates the impact of headache comprehensively.7)
The HIT-68) was developed through the construction of the HIT. The questionnaire can be used as a paper-based test and consists of six questions. The questions are on pain intensity, impact on daily activities, impact on social activities, and mental burden due to headache. There are five choices for each question. Each choice has a predetermined score, and the total score for all six questions is calculated. Based on the total score, the impact of headache on daily living is classified into four grades. The short questionnaire can be completed within one minute. The HIT-6 has been translated into more than 25 languages. The reliability of the Japanese version has also been validated.9)
Questionnaires on patient QOL
(1) Migraine-Specific Quality of Life Questionnaire (MSQ)
(2) Migraine-Specific Quality of Life Measure (MSQOL)
The MSQOL10) is a questionnaire consisting of 25 items developed for the evaluation of the QOL of patients with migraine. High reliability and validity have been reported.
The MSQ ver. 2.111) is composed of 14 items on family, leisure activities, daily activities, work, concentration, tiredness, feeling energetic, canceled work or daily activities, needed help, stopped work or daily activities, social activities, frustration, burden, and afraid. The impact of migraine on QOL is assessed by three dimensions: role function restrictive, role function preventive, and emotional function. The Japanese version of MSQ ver 2.1 has also been evaluated for reliability and validity.12)
Questionnaires on treatment
(1) Migraine Therapy Assessment Questionnaire (MTAQ)
(2) Migraine Assessment of Current Therapy (Migraine-ACT) questionnaire
(3) Migraine Disability Assessment (MIDAS) questionnaire
(4) Headache Impact Test (HIT)
The MTAQ13) is a 9-item questionnaire that requires a response of yes or no to each question. The questionnaire was developed to assess therapeutic effect and identify patients who require changes in treatment.
The Migraine-ACT14) further simplifies the MTAQ. The therapeutic effect and whether the patient need to change treatment can be assessed by answering yes or no to four questions: (1) Does your migraine medication work consistently, in the majority of your attacks? (2) Does the headache pain disappear within 2 hours? (3) Are you able to function normally within 2 hours? (4) Are you comfortable enough with your medication to be able to plan your daily activities? Due to its sensitivity and simplicity, this questionnaire is recommended to be used also in primary care.
Although the MIDAS questionnaire is a tool for evaluating disability, by performing this test before and after treatment, the change in score or grade may indicate the effectiveness of treatment.
For HIT and HIT-6 also, by performing the test before and after treatment, the change in score may indicate treatment efficacy.15)
• References
1) Cady RK, Borchert LD, Spalding W, Hart CC, Sheftell FD: Simple and efficient recognition of migraine with 3-question headache screen. Headache 2004; 44(4): 323-327.
2) Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J, Harrison W: A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology 2003; 61(3): 375-382.
2b) Takeshima T, Sakai F, Suzuki N, Shimizu T, Igarashi H, Araki N, Manaka S, Nakashima K, Hashimoto Y, Iwata M, Fukuuchi Y: A simple migraine screening instrument: validation study in Japan. Japanese Journal of Headache (in press).
3) Stewart WF, Lipton RB, Kolodner K, Liberman J, Sawyer J: Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia 1999; 19(2): 107-114.
4) Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN: Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000; 88(1): 41-52.
5) Iigaya M, Sakai F, Kolodner KB, Lipton RB, Stewart WF: Reliability and validity of the Japanese Migraine Disability Assessment (MIDAS) Questionnaire. Headache 2003; 43(4): 343-352.
6) Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK: PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology 2001; 57(11): 2034-2039.
7) Bayliss MS, Dewey JE, Dunlap I, Batenhorst AS, Cady R, Diamond ML, Sheftell F: A study of the feasibility of Internet administration of a computerized health survey: the headache impact test (HIT). Qual Life Res 2003; 12(8): 953-961.
8) Kosinski M, Bayliss MS, Bjorner JB, Ware JE Jr, Garber WH, Batenhorst A, Cady R, Dahlof CG, Dowson A, Tepper S: A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 2003; 12(8): 963-974.
9) Sakai F, Fukuuchi Y, Iwata M, Hamada J, Igarashi H, Shimizu T, Suyama K, Kageyama S, Arakawa I, Ijiri T, Uechi Y, Nagata T. Evaluation of reliability of the Japanese version “Headache Impact Test (HIT-6)”. Rinsho Iyaku 2004; 20(10): 1045-1054. (In Japanese)
10) Wagner TH, Patrick DL, Galer BS, Berzon RA: A new instrument to assess the long-term quality of life effects from migraine: development and psychometric testing of the MSQOL. Headache 1996; 36(8): 484-492.
11) Martin BC, Pathak DS, Sharfman MI, Adelman JU, Taylor F, Kwong WJ, Jhingran P: Validity and reliability of the migraine-specific quality of life questionnaire (MSQ Version 2.1). Headache 2000; 40(3): 204-215.
12) Sakai F, Fukuuchi Y, Iwata M, Nishimura S, Hamada J, Suzuki N, Igarashi H, Shimizu T, Hashimoto S, Mochizuki A: Reliability and validity of Japanese version of the Migraine-Specific Quality of Life Questionnaire. Shinkei Chiryo 2004; 21(4): 449-458. (In Japanese)
13) Chatterton ML, Lofland JH, Shechter A, Curtice WS, Hu XH, Lenow J, Smullens SN, Nash DB, Silberstein SD: Reliability and validity of the migraine therapy assessment questionnaire. Headache 2002; 42(10): 1006-1015.
14) Dowson AJ, Tepper SJ, Baos V, Baudet F, D’Amico D, Kilminster S: Identifying patients who require a change in their cur-rent acute migraine treatment: the Migraine Assessment of Current Therapy (Migraine-ACT) questionnaire. Curr Med Res Opin 2004; 20(7): 1125-1135.
15) Sakai F, Iwata M, Fukuuchi Y, Suyama K, Motoyama H, Ijiri T, Uechi Y, Nagata T: Improvement of HRQOL by Imigran® tablet (sumatriptan succinate) in patients with migraine. Rinsho Iyaku 2005; 21(1)97-117. (In Japanese)
• Search terms and secondary sources
• Search database: PubMed (2011/9/1)
Headache All fields 54507
& {screening} 23380
& {questionnaire} 1212 & {screener} 10
Migraine All fields 24758
& {screening} 7645
& {questionnaire} 661
& {screener} 9
Cluster headache 2766
& {screening} 1062
& {questionnaire} 63
& {screener} 0
Tension type headache 2416
& {screening} 994
& {questionnaire} 162
& {screener} 0
Primary headache 5549
& {screening} 2795
& {questionnaire} 231
& {screener} 4
• Search database: Ichushi Web for articles published in Japan (2011/12/21)
headache 795 & {questionnaire} 30
& {interview sheet} 88
& {screening}0 & {screener}0
CQ I-11
How is the headache diary used?
Recommendation
The headache diary provides a wealth of information for the management of headache, including the number of days with headache, the number of days of taking medications, and the treatment effect. It is also useful from the viewpoint that it reinforces patient‒physician communication. Use the headache diary in combination with clinical interview is recommended.
| Grade A |
Background and Objective
Patients themselves often do not remember accurately information about their headache, such as the number days with headache, the number of days they have taken medication, and the relation between menstruation and headache. Hence, it is difficult to communicate the information to the physicians. The purpose of the headache diary is to allow the patient to understand the condition of his/her headache and to communicate it effectively to the physician, so as to promote appropriate treatment.
Comments and Evidence
The headache diary provides prospective information of headache, and its usefulness in clinical care and research of headache has been reported.1) Using headache diaries, it is possible to confirm objectively (1) the number days with headache, (2) the property of headache, (3) the intensity of pain, (4) the duration, (5) the accompanying symptoms, (6) the trigger factors, (7) the status of medication use, and (8) the degree of disability.1)-6) Hence for the physicians, their rate of accurately diagnosing individual headaches is increased by using also the headache diary compared to conducting clinical interview alone,7) and they can also monitor the treatment effects. The diagnosis rate of individual headaches is especially high in patients who have headaches in many days, and differentiation between migraine and tension-type headache is possible.8) For the patients, they benefit from being able to monitor their own headache, improvement in drug taking according to the headache type, and improvement in the timing of drug use.5) Moreover, the headache diary is also useful in facilitating patient‒physician communication.9)
When using the headache diary, it is necessary to explain to the patients how it is used and its usefulness, and to obtain their cooperation.
• References
1) Nappi G, Jensen R, Nappi RE, Sances G, Torelli P, Olesen J: Diaries and calendars for migraine. A review. Cephalalgia 2006; 26(8): 905-916.
2) Cohen MJ, McArthur DL: Classification of migraine and tension headache from a survey of 10,000 headache diaries. Headache 1981; 21(1): 25-29.
3) Russell MB, Rasmussen BK, Brennum J, Iversen HK, Jensen RA, Olesen J: Presentation of a new instrument: the diagnostic headache diary. Cephalalgia 1992; 12(6): 369-374.
4) Sakai F, Dobashi K, Igarashi H: Assessing new migraine therapies in Japan. Cephalalgia 1999; 19(Suppl 23): 9-12.
5) Dobashi K, Sakai F, Unno Y: Long-term follow-up of patients with chronic headache using headache diary. Japanese Journal of Headache 1999; 26(1): 17-20. (In Japanese)
6) Stockli HR, Sword A: Zolmitriptan as fast-melt tablet in the acute treatment of patients with migraine attacks: the ZORO study. Praxis (Bern. 1994) 2003; 92(9): 379-389.
7) Jensen R, Tassorelli C, Rossi P, Allena M, Osipova V, Steiner T, Sandrini G, Olesen J, Nappi G; The Basic Diagnostic Head-ache Diary Study Group: A basic diagnostic headache diary (BDHD) is well accepted and useful in the diagnosis of head-ache. A multicentre European and Latin American study. Cephalalgia 2011; 31(15): 1549-1560.
8) Fujita M, Fujihara J: Validity of graphic headache diary for children having primary headaches. Brain and Development 2011; 43(6): 443-447. (In Japanese with English abstract)
9) Sakai F: Application of assist package for diagnosis and treatment. Ministry of Health, Labour and Welfare Grant-in-aids for Scientific Research on Effective Medical Technology Establishment and Promotion. Research Related to Medical Procedures for Headache. Summary and Co-investigator’s Study Reports for 2003. 2004: pp 59-65. (In Japanese)
• Search terms and secondary sources
• Search database: PubMed (2011/12/21)
headache & diary (all field) 504
• Search database: Ichushi Web for articles published in Japan (2011/12/21)
headache & diary (AL) 44
headache & diary 23
CQ I-12
What types of primary headaches require treatment?
Recommendation
The primary headache is a target for treatment if the patient is suffering from it, regardless of the severity. When it is evident that the headache causes disability in daily living, the headache should to be treated aggressively.
| Grade A |
Background and Objective
The prevalence of migraine in Japan is 8.4%, and 74% of the affected persons experience disability in daily living.1) The prevalence of chronic tension-type headache is 1.5%, and 40.5% of the affected persons have disability in daily living.2) The medical facility consultation rate is 30% for migraine, and 73% for chronic tension-type headache.2) However, consulting a medical facility does not guarantee that appropriate treatment is received.
With emphasis being placed on exclusion of secondary headaches, many patients do not receive explanations of the pathophysiology and diagnosis of primary headaches, or receive adequate treatment. Regarding the level of headache care in Japan, reports have indicated that patient needs are not met.3)4) This section examines the primary headaches that should be treated.
Comment and Evidence
The prevalence of migraine in Japan was 8.4%. Among all migraine sufferers, 74% experienced disability in daily living; comprising 4% who frequently required bed rest, 30% who sometimes required bed rest, and 40% who did not require bed rest but had disability.1) Including borderline cases (‘borderline’ tension-type headache with no clinical features of migraine), the prevalence of tension-type headache was 22.3% (including episodic tension-type headache 20.6%, chronic tension-type headache 1.5%), 29.2% of whom had disability in daily living; comprising 0.5% who always required bed rest, 4.7% who frequently required bed rest, and 24% who did not require bed rest but had disability.2) Tension-type headache tends to have a milder impact than migraine. However, for chronic tension-type headache sufferers, 40.5% were affected by disability.2)
The Migraine Disability Assessment (MIDAS) questionnaire5)-7) and the Headache Impact Test (HIT-6)8) are practical tools for the assessment of disability caused by headache. They are used for assessing the degree of disability and for monitoring treatment effectiveness. MIDAS Grade III (score 11) or above, or an HIT-6 score 50 or above indicates moderate or severe disability, and are targets of intensive treatment. In Japan, two reports on the assessment of migraine patients by the MIDAS questionnaire have been published. Igarashi9) evaluated 1,760 nurses or pharmacists with migraine using the MIDAS questionnaire, and reported the distribution of the degree of disability as follows: grade I (minimal or infrequent disability) 63.3%, grade II (mild or infrequent disability) 14.0%, grade III (moderate disability) 8.0%, and grade IV (severe disability) 5.7%. These results are similar to those of an epidemiological survey conducted in France (no response 9.0%).10) According to the study of Iigaya et al.7) on 101 migraine patients who visited a neurological outpatient department, the MIDAS grade distribution was grade I or II 46.5%, grade III 22.2%, and grade IV 31.3%.
The objectives of treating primary headaches are to reduce the headache frequency, headache intensity, and duration; to reduce the time of disability caused by headache and improve QOL; and to prevent the exacerbation of headache by medication overuse. Stratified care according to the degree of disability is recommended for the treatment of acute migraine.11) Stratified care is a treatment approach based on the degree of disability, in which analgesic is prescribed for low-degree disability, while triptan is prescribed from the first treatment for patients with high-degree disability.
• References
1) Sakai F, Igarashi H: Prevalence of migraine in Japan: a nationwide survey. Cephalalgia 1997; 17(1): 15-22.
2) Igarashi H, Sakai F: Epidemiological surgery of tension-type headache. Japanese Journal of Headache 1998; 25(1): 17-19. (In Japanese)
3) Negoro K, Tada Y: Status and roles of headache clinic. Ministry of Health, Labour and Welfare Grants-in-aid for Scientific Research on Effective Medical Technology Establishment and Promotion. Research Related to Medical Procedures for Headache. Summary and Co-investigator’s Study Reports for 2003. 2004: pp 34-39. (In Japanese)
4) Takeshima T, Ishizaki K, Fukuhara Y, Ijiri T, Kusumi M, Wakutani Y, Mori M, Kawashima M, Kowa H, Adachi Y, Urakami K, Nakashima K: Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache 2004; 44(1): 8-19.
5) Stewart WF, Lipton RB, Kolodner K, Liberman J, Sawyer J: Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia 1999; 19(2): 107-114.
6) Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN: Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000; 88(1): 41-52.
7) Iigaya M, Sakai F, Kolodner KB, Lipton RB, Stewart WF: Reliability and validity of the Japanese Migraine Disability Assessment (MIDAS) Questionnaire. Headache 2003; 43(4): 343-352.
8) Sakai F, Fukuuchi Y, Iwata M, Hamada J, Igarashi H, Shimizu T, Suyama K, Kageyama S, Arakawa I, Ijiri T, Uechi Y, Nagata T. Evaluation of reliability of the Japanese version “Headache Impact Test (HIT-6)”. Rinsho Iryo 2004; 20(10): 1045-1054. (In Japanese)
9) Igarashi H: Survey of the status of chronic headache in nurses and pharmacists. Ministry of Health, Labour and Welfare Grants-in-aid for Scientific Research on Effective Medical Technology Establishment and Promotion. Research Related to Medical Procedures for Headache. Summary and Co-investigator’s Study Reports for 2003. 2004: pp 66-74. (In Japanese)
10) Henry P, Auray JP, Gaudin AF, Dartigues JF, Duru G, Lanteri-Minet M, Lucas C, Pradalier A, Chazot G, El Hasnaoui A: Prevalence and clinical characteristics of migraine in France. Neurology 2002; 59(2): 232-237.
11) Lipton RB, Stewart WF, Stone AM, Lainez MJ, Sawyer JP; Disability in Strategies of Care Study group: Stratified care vs step care strategies for migraine: The Disability in Strategies of Care (DISC) Study: A randomized trial. JAMA 2000; 284(20): 2599-2605.
• Search terms and secondary sources
• Search database: PubMed (2011/11/8)
Chronic headache OR Primary headache
& {strategy OR stratified} 484
CQ I-13
What types of primary headache require hospitalized treatment and what are the treatment methods?
Recommendation
The primary headaches that require hospitalized treatment include (1) when life-threatening secondary headache cannot be excluded; (2) rare headaches that require diagnosis and treatment; (3) for the purpose of confirming the efficacy of special treatment; (4) status migrainosus and refractory or chronic cluster headache; and (5) for the purpose of treating medication overuse headache.
| Grade B, C | (admission requirement: B, inpatient treatment: C) |
Background and Objective
Serious life-threatening secondary headache encountered in the emergency outpatient setting obviously require admission and treatment on an inpatient basis. However, the criteria of admitting patients with primary headaches and the treatment methods are decided by individual medical facilities and physicians. There are no clear guidelines.
Comments and Evidence
The evidence level for patients who require admission is grade B, and that for treatment method is grade C. The guidelines on headache management published overseas provide recommendations according to consensus of specialists based on the medical care situation of individual countries or regions. Among these guidelines, the Danish guidelines describe the patients who require hospitalized management, and give the following criteria:
(1) When a serious disease that require immediate treatment is diagnosed
(2) When diagnosis and evaluation of headache are not achieved within the limited time at the outpatient clinic
(3) In the case of rare headache that can be diagnosed by observing a headache attack
(4) In the case of investigating whether a special treatment is effective
(5) For the purpose of stopping medications in severe acute medication overuse cases of migraine and tension-type headache (if outpatient treatment fails, admission for 1-3 weeks2))
In addition, patients with severe status migrainosus and refractory or chronic cluster headache3) who have serious symptoms untreatable in outpatient clinic may desire hospitalized treatment. Regarding hospital treatment methods for these primary headaches, evidence is available for acute-phase treatment of status migrainosus, but evidence is lacking for the treatment of the other headaches. For discontinuing medications on an inpatient basis for patients with severe overuse of acute medications described in (5),4)-7) a metaanalysis on outcome has been conducted by reviewing literature up to 1998.8) Regarding short- and long-term outcome, the 50% headache improvement rate was approximately 80% within 6 months, and 60% over 6 months. However, the types of headaches treated, the types of acute medications, the doses, and the misuse durations varied widely among the articles. There is no clear evidence for treatment method.
• References
1) Guidelines for the management of headache. Danish Neurological Society and the Danish Headache Society. Cephalalgia 1998; 18(1): 9-22.
2) Jauslin P, Goadsby PJ, Lance JW: The hospital management of severe migrainous headache. Headache 1991; 31(10): 658-660.
3) Mather PJ, Silberstein SD, Schulman EA, Hopkins MM: The treatment of cluster headache with repetitive intravenous dihydroergotamine. Headache 1991; 31(8): 525-532.
4) Grazzi L, Andrasik F, D’Amico D, Leone M, Usai S, Kass SJ, Bussone G: Behavioral and pharmacologic treatment of transformed migraine with analgesic overuse: outcome at 3 years. Headache 2002; 42(6): 483-490.
5) Katsarava Z, Fritsche G, Muessig M, Diener HC, Limmroth V: Clinical features of withdrawal headache following overuse of triptans and other headache drugs. Neurology 2001; 57(9): 1694-1698.
6) Tribl GG, Schnider P, Wober C, Aull S, Auterith A, Zeiler K, Wessely P: Are there predictive factors for long-term outcome after withdrawal in drug-induced chronic daily headache? Cephalalgia 2001; 21(6): 691-696.
7) Suhr B, Evers S, Bauer B, Gralow I, Grotemeyer KH, Husstedt IW: Drug-induced headache: long-term results of stationary versus ambulatory withdrawal therapy. Cephalalgia 1999; 19(1): 44-49.
8) Freitag FG, Lake A 3rd, Lipton R, Cady R, Diamond S, Silberstein S; US Headache Guidelines Consortium, Section on Inpatient Treatment Chairpersons. Inpatient treatment of headache: an evidence-based assessment. Headache 2004; 44(4): 342-360.
• Search terms and secondary sources
• Search database: PubMed (2011/8/9)
#1 primary headache 5527
#2 hospitalization OR inpatient 215346
#3 primary headache and (hospitalization OR inpatient) 104
#4 primary headache and guideline 79;
#5 #3 or #4 181
CQ I-14
How is pharmacotherapy using over-the-counter medications planned?
Recommendation
The choice of pharmacotherapy depends on the severity of headache, the frequency of headache, and the degree of disability. Among the primary headaches, mild headaches can be controlled by over-the-counter (OTC) medications. When the headache is moderate or severe and does not respond to OTC medications, or when OTC medications have been taken frequently, pharmacotherapy under a physician’s guidance is recommended. Physician should set a limit on the number of days of drug taking (not more than 10 days a month) to prevent patients from developing medication-overuse headache, and instruct patients who take medications relatively frequently to choose single-ingredient OTC drugs.
| Grade A |
Background and Objective
Some 40 million persons in Japan are estimated to be affected by chronic headache.1)2) Among 8.4 million persons estimated to be affected by migraine, approximately 74% have disability in daily living, indicating that pharmacotherapy can play a big role. On the other hand, there is a lack of awareness about migraine, and only 2.7% of the migraine sufferers consult a medical facility regularly.1)2) Most of the headache patients presumably manage by taking OTC medications. The major primary headaches comprise migraine, tension-type headache, and cluster headache. OTC medications can be expected to be effective against only mild migraine and episodic tension-type headache. Before starting pharmacotherapy, life-threatening secondary headaches should be excluded.3) Then, OTC medications may be one of the options of pharmacotherapy for migraine and episodic tension-type headache.
Comments and Evidence
Headache is classified by severity and disability into three grades: (1) mild: not disabling; (2) moderate: has impact on daily living or work; and (3) severe: not able to carry out activities of daily living or work, requiring bed rest. Among the primary headaches, when the headache is always mild, afflicts minimal suffering and does not impair daily living, this type of headache can be managed by observation, lifestyle improvement and self-care such as stretching. Even when the headache inflicts suffering, mild cases can be controlled by OTC medications.
For OTC antipyretic analgesics, single-ingredient or combination products approved by the Ministry of Health, Labour and Welfare in Japan are shown in Table 1.4) Acetaminophen 1,000 mg,5) aspirin 1,000 mg,6) and ibuprofen 200 mg and 400 mg7) have been reported to be effective for migraine and tension-type headache.8) A combination preparation of aspirin, acetaminophen and caffeine has been evaluated in a double-blind randomized control trial (RCT) and reported to be effective for migraine.9) This fixed combination preparation has also been reported to be more effective than single substances.10) In recent year, loxoprofen has been added as a switch OTC, but there is no report at RCT level.
Since patients can obtain unlimited OTC medications, it is necessary to explain and draw the patients’ attention to the fact that frequent use for long periods of time may cause medication-overuse headache.
Table 1. Ingredients of OTC antipyretic analgesics.
| Antipyretic analgesic ingredients | Aspirin, acetaminophen, isopropylantipyrine, ibuprofen, ethenzamide |
| Sedative hypnotic ingredients | Enhancement of analgesic effect of antipyretic analgesic, also sedative effect |
| Allylisopropylacetylurea, bromvalerylurea | |
| Antacid ingredient | Suppresses gastric discomfort due to antipyretic analgesic ingredients |
| Herbal medicine ingredients | Exhibit antipyretic effect (Jiryu, etc.) and analgesic effect (Shakuyaku) |
| Other ingredients | Supplement the analgesic effect of antipyretic analgesics |
| Anhydrous caffeine |
According to the criteria for medication-overuse headache described in the International Classification of Headache Disorders 3rd Edition (beta version) published in Cephalalgia in June 2013,11) medication-overuse headache is diagnosed when headache is associated with regular intake of simple analgesic on ≥15 days per month or combination analgesics on ≥10 days per month for >3 months. Therefore, for patients who are taking OTC medications on ≥10 days per month and patients who have been taking OTC medications despite no response, a change to acute medications according to physicians’ instructions and administration of prophylactic medications should be considered.
• References
1) Sakai F: [Headache/Pain] Epidemiology and medical economics of headache. Advances in Neurological Sciences 2002; 46(3): 343-349. (In Japanese)
2) Sakai F, Igarashi H: Prevalence of migraine in Japan: a nationwide survey. Cephalalgia 1997; 17(1): 15-22.
3) Morimatsu M: [Focusing on Treatment Guideline for Chronic Headache] Diagnostic testing review: on the classification and differential diagnosis of chronic headache. Kongetsu No Chiryo 2003; 11(6): 641-644. (In Japanese)
4) Hori M (supervising editor), Fukuo Y (clinical supervising editor), Iryo Joho Kenkyujo, SIC Corporation (Ed.): OTC Drug Guidebook, 2nd Edition. Jiho, 2009. (In Japanese)
5) Derry S, Moore RA, McQuay HJ: Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev 2010; (11): CD008040.
6) Kirthi V, Derry S, Moore RA, McQuay HJ: Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev 2010; (4): CD008041.
7) Suthisisang C, Poolsup N, Kittikulsuth W, Pudochakan P, Wiwatpanich P: Efficacy of low-dose ibuprofen in acute migraine treatment: systematic review and meta-analysis. Ann Pharmacother 2007; 41(11): 1782-1791.
8) Haag G, Diener HC, May A, Meyer C, Morck H, Straube A, Wessely P, Evers S; DMKG; DGN; OKSG; SKG: Self-medication of migraine and tension-type headache: summary of the evidence-based recommendations of the Deutsche Migrane und Kopfschmerzgesellschaft (DMKG), the Deutsche Gesellschaft fur Neurologie (DGN), the Osterreichische Kopf-schmerzgesellschaft (OKSG) and the Schweizerische Kopfwehgesellschaft (SKG). J Headache Pain 2011; 12(2): 201-217.
9) Lipton RB, Stewart WF, Ryan RE Jr, Saper J, Silberstein S, Sheftell F: Efficacy and safety of acetaminophen, aspirin, and caffeine in alleviating migraine headache pain: three double-blind, randomized, placebo-controlled trials. Arch Neurol 1998; 55(2): 210-217.
10) Diener HC, Pfaffenrath V, Pageler L, Peil H, Aicher B: The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study. Cephalalgia 2005; 25(10): 776-787.
11) Headache Classification Committee if the International Headache Society (IHS): The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33(9): 629-808.
• Search terms and secondary sources
• Search database: PubMed (2011/12/21)
Headache & OTC 50 & aspirin 530
& acetaminophen 372
& ibuprophen 185
& self-medication 371
& guideline 405
• Search database: Ichushi Web for articles published in Japan (2011/12/21)
Headache &OTC 58
& aspirin194
& acetaminophen165
& ibuprofen96
& pharmacy 138
& pharmacist 180
CQ I-15
Are herbal medicines (Kampo) effective?
Recommendation
Based on traditional medicine, herbal medicine (Kampo) is a treatment that had been used empirically. Various herbal medicines have been used empirically for headache, and have shown effects. Scientific evidence has been accumulated in recent years, and the effectiveness for headache is being proven.
| Grade B |
Background and Objective
Since herbal medicine (Kampo) is a treatment that was developed through empirical use, it cannot be denied that scientific evidence such as basic and clinical research remains insufficient. In this section, the effectiveness of Kampo is examined by reviewing articles with evidence level of case series or above.
Comments and Evidence
Comments are given below by Kampo formula.
1. Goshuyuto (呉茱萸湯 in Japanese, Evodia Decoction in English)
One report of double-blind randomized controlled trial (DB-RCT) on responders only, one report of randomized controlled (open label, cross-over) trial, one report of comparative study between Kampo formulas, and two reports of case series were identified. Taking into account the prescription system of Kampo medicines, Odaguchi et al.1) conducted a DB-RCT on a selected subgroup of 53 patients with chronic headache who responded to Goshuyuto, and observed significant decrease in headache frequency and decrease in frequency of analgesic intake. According to the Kampo prescription system, even with the same diagnosis of migraine, different Kampo formulas may be prescribed depending on the constitution of individuals. Therefore, it is difficult to conduct conventional clinical research such as DR-RCT on Kampo prescriptions. Maruyama2) conducted an open-label crossover study of Goshuyuto and lomerizine hydrochloride in patients with migraine. Despite limitations of a relatively small number of cases and short wash-out period, the study showed higher efficacy of Goshuyuto compared to lomerizine hydrochloride. Seki et al.3) and Maeda et al.4) used Goshuyuto for chronic headache, and observed high improvement rates of 79.5% and 89%, respectively. Especially, Maeda et al.4) reported high improvement rate in vascular headache patients with severe pain, and the effect appeared mostly within 2 weeks, suggesting that early effectiveness may be expected for migraine. Akamine et al.5) reported effectiveness in 76.7% of patients with tension-type headache. Thus, Goshuyuto is highly effective for both migraine and tension-type headache.
2. Keishininjinto (桂枝人参湯 in Japanese, Cinnamon Twig and Ginseng Decoction in English)
One report of randomized controlled study comparing with Goshuyuto and one report of non-randomized crossover study between Keishininjinto and Chotosan were identified. In the randomized controlled study for chronic headache comparing with Goshuyuto,3) Keishininjinto was used as a comparator for Goshuyuto and showed an improvement rate of 61.4%. In the crossover study between Keishininjinto and Chotosan for chronic headache,6) the number of cases in which Keishininjinto was more useful tended to be greater although there was no significant difference.
3. Chotosan (釣藤散 in Japanese, Uncaria Decoction in English)
One report of non-randomized crossover study between Chotosan and Keishininjinto and five reports of case series were identified. In the non-randomized crossover study between Chotosan and Keishininjinto for chronic headache, Chotosan was effective although the number of effective cases was slightly smaller compared to Keishininjinto.6) In a case series of 54 cases of chronic headache, the improvement rate was 74.1%.7) In two case series of chronic tension-type headache, high improvement rates of 94% in 150 cases8) and 70% in 20 cases9) were reported. Unfortunately, the evidence level of these reports is low because the time of symptom improvement was unclear and the age group was biased. In a study on chronic headache caused by intracranial organic disease, 80% of the patients showed slight improvement or better.10) In another study on chronic headache caused by cerebrovascular disease, slight or better improvement was shown in 78.3% of the patients and effectiveness was observed within 4 to 7 weeks in nearly 70%.11)
4. Kakkonto (葛根湯 in Japanese, Kudzu Decoction in English)
One case series report of 23 cases of chronic tension-type headache not sufficiently treated by anxiolytic medication was identified.12) Improvement rates of 50% for headache and 60.9% for heavy headiness were reported. Kakkonto is conventionally taken short-term or on an as-needed basis. However, in this study, the period of intake was not fixed. Among the patients, some used the formula for more than one month and there was one case of adverse event including gastric discomfort. Study design based on the conventional usage of Kampo formulas should be considered.
5. Goreisan (五苓散 in Japanese, Five Ingredient Powder with Poria in English)
Two reports of case series for headache accompanying hemodialysis were identified. Although the method of intake lacked consistency, significant improvement of headache as assessed by VAS score was observed in 11 hemodialysis patients with headache.13) In another questionnaire study on 16 hemodialysis patients, “marked response” or “response” was obtained in 12 patients.14) Headache associated with hemodialysis may be caused by transient cerebral edema. According to the pharmacological study conducted by Isohama,15) Goreisan regulates water metabolism by acting on aquaporin (AQP) in cell membrane. Especially, AQP4 is involved in cerebral edema, and Goreisan has been shown to suppress AQP4. Clinical use of Goreisan for chronic subdural hematoma has been reported but only as case report.
As shown by the above reports, evidence at a level of case series or above is available for only five Kampo formulas. Only two studies have high evidence level; a DB-RCT and an open-label crossover study for Goshuyuto. Almost all the other reports are case series. One of the reasons is that the prescription system for Kampo formula is that “even for the same diagnosis, prescription differs depending on constitution”, and this feature hampers research development. In the future, development of study design that is adapted to Kampo prescription system is necessary.
• References
1) Odaguchi H, Wakasugi A, Ito H, Shoda H, Gono Y, Sakai F, Hanawa T: The efficacy of goshuyuto, a typical Kampo (Japanese herbal medicine) formula, in preventing episodes of headache. Curr Med Res Opin 2006; 22(8): 1587-1597.
2) Maruyama T: Study of the usefulness of goshuyuto for migraine prevention: open crossover study with lomerizine hydrochloride. Pain and Kampo Medicine 2006; 16: 30-39. (In Japanese)
3) Seki H, Okita N, Takase T, Matsuda K: The effect of goshuyuto for chronic headache: comparison with Keishininjinto using an envelope method. Pharma Medica 1993; 11(12): 288-291. (In Japanese)
4) Maeda H, Miyagi A, Sugawara T: The effect of goshuyuto for chronic headache. Science of Kampo Medicine 1998; 22(2): 53-57. (In Japanese)
5) Akamine M, Hyodo Y, Ashihara M, Sata A, Tsutsumi M, Kosugi M, Moriyama H, Oota R: The usefulness of goshuyuto for tension-type headache. Journal of Japanese Association of Oriental Psychosomatic Medicine 2000; 15(1/2): 36-38. (In Japanese)
6) Matsumoto H, Kashiwagi M, Matsutani M, Chiba S, Hashimoto S, Narasaki Y, Ohshima T, Ito K, Yatsushiro N, Uchiyama H: The usefulness of Keishininjinto and Chotosan for chronic headache. The Japanese Journal of Clinical and Experimental Medicine 1995; 72(5): 1299-1303. (In Japanese)
7) Sadato A, Orita Y, Kikuchi H, Ishikawa J, Tsuda N, Higashi K, Yamamoto T, Kondo Y: The effect of Chotosan (TJ-47) for chronic headache. Neurosurgery Letters 1992; 2(2): 171-176. (In Japanese)
8) Nagata K: Clinical effect of Tsumura Chotosan for chronic tension-type headache. JAMA <Japanese edition> 1996; 17(11): 38-39. (In Japanese)
9) Takada S: The effectiveness of Chotosan for chronic tension-type headache. Science of Kampo Medicine 1998; 22(4): 121-124. (In Japanese)
10) Fukushima T, Tomonaga M, Tanaka S, Mizoguchi T, Oota T, Ikui H, Yamashita M, Hirakawa T, Matsumoto N: Clinical effect of Chotosan for headache. Science of Kampo Medicine 1994; 18(8): 272-275. (In Japanese)
11) Kimura I, Sasao S: Clinical effect of Tsumura Chotosan for chronic headache in patients with cerebrovascular disease. Geriat Med 1989; 27(3): 445-449. (In Japanese)
12) Yamamoto T: Clinical effect of Kakkonto for chronic tension headache caused by stiff shoulder. The Japanese Journal of Clinical and Experimental Medicine 1995; 72(8): 2085-2088. (In Japanese)
13) Noguchi T: Therapeutic effect of Goreisan for headache accompanying hemodialysis. Science of Kampo Medicine 2010; 34(2): 182-183. (In Japanese)
14) Muroga K: Oriental medical treatment for headache in maintenance dialysis patients. Oriental Medicine 1999; 27(2): 46-47. (In Japanese)
15) Isohama Y: Regulatory mechanism of water metabolism by Goreisan via aquaporin. Science of Kampo Medicine 2011; 35(2): 186-189. (In Japanese)
• Search terms and secondary sources
• Search database: Ichushi Web for articles published in Japan (2011/10/24)
{kampo}or {Kampo medicine}
& headache}or {migraine}or {tension headache}or {chronic headache}1213
• Search database: PubMed (2011/10/24)
{kampo} or {herbal medicine} or {traditional medicine} or {oriental medicine}
& {headache} or {migraine} 387
CQ I-16
What other therapies are available, apart from pharmacotherapy?
Recommendation
Apart from pharmacotherapy, other therapies for primary headaches include behavioral therapy, physical therapy and supplements. Because these therapies are not covered by health insurance, and some adverse events have also been reported, use of these therapies should consider the characteristics of individual patients and also accountability. Details of non-pharmacotherapy for migraine and tension-type headache can be found in the respective sections.
| Grade B, C | (depending on therapy) |
Background and Objective
Other than pharmacotherapy, other prophylactic treatments have been anticipated to be effective for primary headaches. A literature search was conducted focusing on non-pharmacotherapies that have been tested by randomized controlled trials (RCT).
Comments and Evidence
Treatments for primary headaches other than pharmacotherapy include the following:
(1) Behavioral therapy: Relaxation training, biofeedback, cognitive-behavioral therapy, and hypnosis → <migraine, tension-type headache>
(2) Physical therapy: Acupuncture, transcutaneous electrical nerve stimulation → <migraine, tension-type headache>
(3) Supplements: Feverfew, magnesium, vitamin B2 (riboflavin) → <migraine>
These are therapeutic options for patients who prefer nonpharmacologic treatment, patients with poor tolerance to pharmacologic treatments, patients with medical contraindications for pharmacologic treatments, patients showing no response to pharmacologic treatment, patients who are pregnancy or planning pregnancy, patients with a history of medication overuse headache, and patients with significant stress.1)
(1) Behavioral therapy
Relaxation training, thermal biofeedback combined with relaxation training, electromyogram biofeedback, cognitive-behavioral therapy, and hypnosis are useful prophylactic treatments for tension-type headache and migraine.1)2) A meta-analysis revealed that relaxation and biofeedback training improved migraine in over 20% of the patients.3) Clinical improvement may be expected by a combination of prophylactic pharmacotherapy and relaxation/feedback training (recommendation grade B). In recent years, cognitive-behavioral therapy has been reported to be effective especially for migraine in children (recommendation grade B).4)
(2) Physical therapy
Acupuncture and transcutaneous electrical nerve stimulation as acute and prophylactic treatments for primary headaches have been tested by RTCs and reported to be effective. However, these trials lack quality and quantity. Further evidence has to be accumulated5)-7) (recommendation grade B).
RCTs of chiropractic and spinal manipulation have been reported.8)9) However, the opinions of experts are divided. Since risk is involved depending on the manipulation, caution has to be exercised when used in therapy (recommendation grade C).
(3) Supplements
Feverfew, magnesium, vitamin B2 (riboflavin) have been reported to be effective prophylactic agents for migraine10)-12) (recommendation grade B).
• References
1) Silberstein SD: Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6): 754-762.
2) Pryse-Phillips WE, Dodick DW, Edmeads JG, Gawel MJ, Nelson RF, Purdy RA, Robinson G, Stirling D, Worthington I: Guidelines for the nonpharmacologic management of migraine in clinical practice. Canadian Headache Society. CMAJ 1998; 159(1): 47-54.
3) Holroyd KA, Penzien DB: Pharmacological versus non-pharmacological prophylaxis of recurrent migraine headache: a meta-analytic review of clinical trials. Pain 1990; 42(1): 1-13.
4) Trautmann E, Kroner-Herwig B: A randomized controlled trial of Internet-based self-help training for recurrent headache in childhood and adolescence. Behav Res Ther 2010; 48(1): 28-37.
5) Melchart D, Linde K, Fischer P, White A, Allais G, Vickers A, Berman B: Acupuncture for recurrent headaches: a systematic review of randomized controlled trials. Cephalalgia 1999; 19(9): 779-786 ; discussion 765.
6) Allais G, De Lorenzo C, Quirico PE, Airola G, Tolardo G, Mana O, Benedetto C: Acupuncture in the prophylactic treatment of migraine without aura: a comparison with flunarizine. Headache 2002; 42(9): 855-861.
7) Ahmed HE, White PF, Craig WF, Hamza MA, Ghoname ES, Gajraj NM: Use of percutaneous electrical nerve stimulation (PENS) in the short-term management of headache. Headache 2000; 40(4): 311-315.
8) Tuchin PJ, Pollard H, Bonello R: A randomized controlled trial of chiropractic spinal manipulative therapy for migraine. J Manipulative Physiol Ther 2000; 23(2): 91-95.
9) Bronfort G, Assendelft WJ, Evans R, Haas M, Bouter L: Efficacy of spinal manipulation for chronic headache: a systematic review. J Manipulative Physiol Ther 2001; 24(7): 457-466.
10) Peikert A, Wilimzig C, Kohne-Volland R: Prophylaxis of migraine with oral magnesium: results from a prospective, multi-center, placebo-controlled and double-blind randomized study. Cephalalgia 1996; 16(4): 257-263.
11) Boehnke C, Reuter U, Flach U, Schuh-Hofer S, Einhaupl KM, Arnold G: High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol 2004; 11(7): 475-477.
12) Pittler MH, Ernst E: Feverfew for preventing migraine. Cochrane Database Syst Rev 2004; (1): CD002286.
• Search terms and secondary sources
• Search database: PubMed (2013/3/11)
headache (All Fields) 59278
headache (All Fields) & alternative medicine 1758 +Limits1 (English, Randomized Controlled Trial, Human) 392
& acupuncture 479 +Limits1 0
& biofeedback 561 +Limits1 0
&chiropractic 176 +Limits1 0
& hypnosis 161 +Limits1 0
& herbal medicine 103 +Limits1 0
• Search database: Ichushi Web for articles published in Japan
(headache /TH or headache /AL) 5405
& (alternative medicine/TH or alternative therapy/AL) 183
CQ I-17
Is cognitive-behavioral therapy effective for primary headaches?
Recommendation
As a non-pharmacotherapy for primary headaches, cognitive-behavioral therapy has been evaluated by randomized controlled trials in European and American countries, and the therapeutic effect has been confirmed. Using cognitive-behavioral therapy, headache can be ameliorated in 30 to 50% of the patients and therapeutic effect comparable to pharmacotherapy may be expected. The therapeutic effect increases when cognitive behavioral therapy is combination with pharmacotherapy. However, the number of facilities in Japan offering cognitive-behavioral therapy for headache is limited.
| Grade B |
Background and Objective
The cognitive-behavioral therapeutic approach for primary headaches has been conducted since more than 30 years ago. Most of the previous research studied relaxation (including stress management), biofeedback therapy and cognitive therapy, either alone or in combination. These therapies are grouped together and called cognitive-behavioral therapy. Although there are reports on relaxation alone and biofeedback therapy alone, there are few reports on cognitive therapy alone in the literature.
In this section, the usefulness of cognitive behavioral therapy for primary headaches is presented.
Comments and Evidence
Validation of the usefulness of cognitive-behavioral therapy for primary headaches has been conducted mainly in European and American countries, and many randomized controlled trials have reported the usefulness of this therapy.1)-8) For tension-type headache, headache reduction rates of 37 to 50% have been reported,9) and cognitive-behavioral therapy has been reported to have equivalent therapeutic effect as amitriptyline.3) For migraine also, cognitive-behavioral therapy reduced headache by 32 to 49%, and a combination of relaxation and biofeedback therapy achieved equivalent prophylactic effect as propranolol and even better long-term effectiveness than propranolol.6) Apart from randomized controlled trials, several metaanalyses and reviews showing the effectives of behavioral therapy have been reported.9)-13) As of present, cognitive-behavioral therapy has been shown to be effective for migraine and tension-type headache, but little therapeutic effect for cluster headache.9)
For primary headaches, the therapeutic effect is further improved when cognitive-behavioral therapy is used in combination with pharmacotherapy, compared with cognitive-behavioral therapy alone.6)14)15) Furthermore, superior and long-lasting clinical effect has been reported for cognitive-behavioral therapy (biofeedback therapy) in children, and the efficacy is even higher than in adults.11)16)
However, in a survey in which therapy was administered by persons who were not experienced in behavioral therapy, no significant difference in clinical improvement rate was observed, suggesting an issue in using this therapy as regular treatment in routine clinical setting.17) Another report found no difference in efficacy between relaxation alone and combined relaxation and biofeedback therapy.18)
Behavioral therapy has several merits: no risk of drug dependence because it is a non-pharmacotherapy, very few adverse events, and low cost. On the other hand, the demerits include inconsistent methods used in different facilities due to the lack of standardized method, requirement of therapists to possess certain level of knowledge and skills, and lack of immediate response. Recent research is heading for the direction of examining the effectiveness and impact on medical cost by comparing or combining with representative existing pharmacologic treatments.3)
• References
1) Holroyd KA, O’Donnell FJ, Stensland M, Lipchik GL, Cordingley GE, Carlson BW: Management of chronic tension-type headache with tricyclic antidepressant medication, stress management therapy, and their combination: a randomized con-trolled trial. JAMA 2001; 285(17): 2208-2215.
2) Bussone G, Grazzi L, D’Amico D, Leone M, Andrasik F: Biofeedback-assisted relaxation training for young adolescents with tension-type headache: a controlled study. Cephalalgia 1998; 18(7): 463-467.
3) Holroyd KA, Nash JM, Pingel JD, Cordingley GE, Jerome A: A comparison of pharmacological (amitriptyline HCL) and nonpharmacological (cognitive-behavioral) therapies for chronic tension headaches. J Consult Clin Psychol 1991; 59(3): 387-393.
4) D’Souza PJ, Lumley MA, Kraft CA, Dooley JA: Relaxation training and written emotional disclosure for tension or migraine headaches: a randomized, controlled trial. Ann Behav Med 2008; 36(1): 21-32.
5) Kropp P, Gerber WD, Keinath-Specht A, Kopal T, Niederberger U: Behavioral treatment in migraine. Cognitive-behavioral therapy and blood-volume-pulse biofeedback: a cross-over study with a two-year follow-up. Funct Neurol 199: 12(1): 17-24.
6) Kaushik R, Kaushik RM, Mahajan SK, Rajesh V: Biofeedback assisted diaphragmatic breathing and systematic relaxation versus propranolol in long term prophylaxis of migraine. Complement Ther Med 2005; 13(3): 165-174.
7) Richardson GM, McGrath PJ: Cognitive-behavioral therapy for migraine headaches: a minimal-therapist-contact approach versus a clinic-based approach. Headache 1989; 29(6): 352-357.
8) Martin PR, Forsyth MR, Reece J: Cognitive-behavioral therapy versus temporal pulse amplitude biofeedback training for recurrent headache. Behav Ther 2007; 38(4): 350-363.
9) Penzien DB, Rains JC, Andrasik F: Behavioral management of recurrent headache: three decades of experience and empiricism. Appl Psychophysiol Biofeedback 2002; 27(2): 163-181.
10) Holroyd KA, Penzien DB: Pharmacological versus non-pharmacological prophylaxis of recurrent migraine headache: a meta-analytic review of clinical trials. Pain 1990; 42(1): 1-13.
11) Sarafino EP, Goehring P: Age comparisons in acquiring biofeedback control and success in reducing headache pain. Ann Behav Med 2000; 22(1): 10-16.
12) Bogaards MC, ter Kuile MM: Treatment of recurrent tension headache: a meta-analytic review. Clin J Pain 1994; 10(3): 174-190.
13) Penzien DB, Rains JC, Lipchik GL, Creer TL: Behavioral interventions for tension-type headache: overview of current therapies and recommendation for a self-management model for chronic headache. Curr Pain Headache Rep 2004; 8(6): 489-499.
14) Andrasik F: Biofeedback in headache: an overview of approaches and evidence. Cleve Clin J Med 2010; 77(Suppl 3): S72-76.
15) Nicholson RA, Buse DC, Andrasik F, Lipton RB: Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr Treat Options Neurol 2011; 13(1): 28-40.
16) Trautmann E, Kroner-Herwig B: A randomized controlled trial of Internet-based self-help training for recurrent headache in childhood and adolescence. Behav Res Ther 2010; 48(1): 28-37.
17) Fichtel A, Larsson B: Relaxation treatment administered by school nurses to adolescents with recurrent headaches. Headache 2004; 44(6): 545-554.
18) Mullally WJ, Hall K, Goldstein R: Efficacy of biofeedback in the treatment of migraine and tension type headaches. Pain Physician 2009; 12(6): 1005-1011.
• Search terms and secondary sources
• Search database: PubMed (2011/12/1)
Cognitive-behavioral therapy 44110
& {headache OR tension-type headache OR migraine} 553
{Cognitive-behavioral therapy}
OR {biofeedback} OR {relaxation} 98049
& tension-type headache 128
{Cognitive-behavioral therapy}
OR {biofeedback} OR {relaxation}
& migraine 405
Limits: English, Randomized Controlled Trial, Humans 38
CQ I-18
Does anxiety/depression coexist with primary headaches?
Recommendation
Patients with migraine and tension-type headache tend to develop psychological states such as anxiety and depression as a level of symptom, and these psychological states are associated with chronicity of headache. In addition, psychiatric disorders such as mood disturbances (major depression) and anxiety disorder (including panic disorder) are common comorbidities. Paying attention to the coexistence of these psychological states and psychiatric disorders is clinically important.
| Grade B |
Background and Objective
Psychological factors such as anxiety and depression have been known to be closely associated with the onset and progression of migraine and tension-type headache. In addition, many studies have reported various psychiatric disorders that tend to be coexist with primary headaches, such as mood disturbances (such as major depression, dysthymia, and bipolar disorder), drug addiction, anxiety disorders (panic disorder, phobia, generalized anxiety disorder), somatoform disorder (such as somatization disorder, and pain disorder). Especially, the involvement of abnormal serotonin metabolism in the relationship between migraine and panic disorder or major depression has gained attention.
The objective of this section is to collect available literature and present the knowledge concerning the relationship between primary headaches and depression or anxiety.
Comment and Evidence
For migraine, large epidemiological surveys have been conducted actively. The annual prevalence of major depression among migraine patients has been reported to be approximately 8.6%,1) with odds ratio of 2.2.2) The lifetime prevalence of major depression in migraine patients has been reported to range from 18 to 40%, and many epidemiological studies have shown odds ratios of 3 to 4.1)3)-6) The relations between migraine and various anxiety disorders such as panic disorder, generalized anxiety disorder, phobia, and obsessive-compulsive disorder have been studied. High odds ratios for panic disorder ranging from 2.8 to 6.0 have been reported,1)3)6)-8) similar to depression. Many studies so far have demonstrated an association of migraine with major depression, panic disorder, and phobia, but no significant relationship with obsessive-compulsive disorder and substance abuse. Psychiatric comorbidities in migraine patients in headache centers also showed similar high association9)-11) as in epidemiological studies. The prevalence of psychiatric comorbidities in migraine patients is especially high in migraine with aura, chronic migraine, and migraine with medication overuse.12)
Research on the association of psychosocial factors and psychiatric disorders with tension-type headache is less abundant than that with migraine. The association with psychological states including psychosocial stress and anxiety/depression has been found, and psychiatric comorbidities including mood disturbances (such as depression), anxiety disorders (such as panic disorder, generalized anxiety disorder, and obsessive-compulsive disorder), and somatoform disorders have also been reported9)10)13) as in migraine.
Various tools have been used for the screening of these psychiatric disorders, such as SDS, HAM-A, HAM-D, and the Hospital Anxiety and Depression Scale (HADS). The reliability and validity of HADS for the evaluation of primary headaches have been studied.14)-17)
Almost all previous reports have pointed out a relationship between primary headaches and anxiety or depression. However, the evaluation methods for psychological states or psychiatric disorders are not standardized. While the high association has been attributed to the involvement of serotonin, a consensus is yet to be arrived.
• References
1) Jette N, Patten S, Williams J, Becker W, Wiebe S: Comorbidity of migraine and psychiatric disorders: a national population-based study. Headache 2008; 48(4): 501-516.
2) Antonaci F, Nappi G, Galli F, Manzoni GC, Calabresi P, Costa A: Migraine and psychiatric comorbidity: a review of clinical findings. J Headache Pain 2011; 12(2): 115-125.
3) Breslau N, Davis GC, Andreski P: Migraine, psychiatric disorders, and suicide attempts: an epidemiologic study of young adults. Psychiatry Res 1991; 37(1): 11-23.
4) Breslau N, Schultz LR, Stewart WF, Lipton RB, Lucia VC, Welch KM: Headache and major depression: is the association specific to migraine? Neurology 2000; 54(2): 308-313.
5) Mitsikostas DD, Thomas AM: Comorbidity of headache and depressive disorders. Cephalalgia 1999; 19(4): 211-217.
6) Swartz KL, Pratt LA, Armenian HK, Lee LC, Eaton WW: Mental disorders and the incidence of migraine headaches in a community sample: results from the Baltimore Epidemiologic Catchment area follow-up study. Arch Gen Psychiatry 2000; 57(10): 945-950.
7) Stewart W, Breslau N, Keck PE Jr: Comorbidity of migraine and panic disorder. Neurology 1994; 44(10 Suppl 7): S23-27.
8) Breslau N, Schultz LR, Stewart WF, Lipton R, Welch KM: Headache types and panic disorder : directionality and specificity. Neurology 2001; 56(3): 350-354.
9) Beghi E, Bussone G, D’Amico D, Cortelli P, Cevoli S, Manzoni GC, Torelli P, Tonini MC, Allais G, De Simone R, D’Onofrio F, Genco S, Moschiano F, Beghi M, Salvi S: Headache, anxiety and depressive disorders: the HADAS study. J Headache Pain 2010; 11(2): 141-150.
10) Puca F, Genco S, Prudenzano MP, Savarese M, Bussone G, D’Amico D, Cerbo R, Gala C, Coppola MT, Gallai V, Firenze C, Sarchielli P, Guazzelli M, Guidetti V, Manzoni G, Granella F, Muratorio A, Bonuccelli U, Nuti A, Nappi G, Sandrini G, Verri AP, Sicuteri F, Marabini S: Psychiatric comorbidity and psychosocial stress in patients with tension-type headache from headache centers in Italy. The Italian Collaborative Group for the Study of Psychopathological Factors in Primary Headaches. Cephalalgia 1999; 19(3): 159-164.
11) Guidetti V, Galli F, Fabrizi P, Giannantoni AS, Napoli L, Bruni O, Trillo S: Headache and psychiatric comorbidity: clinical aspects and outcome in an 8-year follow-up study. Cephalalgia 1998; 18(7): 455-462.
12) Radat F, Swendsen J: Psychiatric comorbidity in migraine: a review. Cephalalgia 2005; 25(3): 165-178.
13) Holroyd KA, Stensland M, Lipchik GL, Hill KR, O’Donnell FS, Cordingley G: Psychosocial correlates and impact of chronic tension-type headaches. Headache 2000; 40(1): 3-16.
14) Wacogne C, Lacoste JP, Guillibert E, Hugues FC, Le Jeunne C: Stress, anxiety, depression and migraine. Cephalalgia 2003; 23(6): 451-455.
15) Zwart JA, Dyb G, Hagen K, Odegard KJ, Dahl AA, Bovim G, Stovner LJ: Depression and anxiety disorders associated with headache frequency. The Nord-Trondelag Health Study. Eur J Neurol 2003; 10(2): 147-152.
16) Juang KD, Wang SJ, Lin CH, Fuh JL: Use of the hospital anxiety and depression scale as a screening tool for patients with headache. Zhonghua Yi Xue Za Zhi (Taipei) 1999; 62(11): 749-755.
17) Devlen J: Anxiety and depression in migraine. J R Soc Med 1994; 87(6): 338-341.
• Search terms and secondary sources
• Search database: PubMed (2011/12/1)
Major depression 93622
& {migraine} 389
& {tension type headache} 1668
& {headache} 12274 Panic disorder 9209
& {migraine} 105
& {tension type headache} 12
& {headache} 109
CQ I-19
How should occupational health physicians and brain health check-up physicians manage headache?
Recommendation
Occupational health physicians and brain health check-up physicians should participate actively in providing headache medical care for workers and health check-up receivers with headaches.
| Grade A |
Background and Objective
The objective of this section is to search for literature on the frequency and status of persons with primary headaches in the workplace and brain health check-up setting to examine the roles of occupational health physicians and brain health check-up physicians in providing medical care for headache.
Comment and Evidence
Migraine has been reported to cause reduction in working hours and socio-economic loss.1) According to a survey of primary headaches in the workplace, the prevalence of migraine was 13.2% (male 11.6%, female 26.6%), episodic tension-type headache 29.15% (male 27.6%, female 41.3%), and chronic tension-type headache 0.9% (male 0.8%, female 1.3%). Although the majority (84.3%) of workers suffering from migraine reported decrease in working efficiency, the rate of consulting a medical facility was as low as 23.7%.2) In a study on persons receiving brain health check-up, the prevalence of migraine was 10.2% (male 6.1%, female 19.4%). Most (75.4%) were mild cases, and the rate of consulting a medical facility was also low at 15.1%. The rates of medical facility consultation were 9.8% among persons suffering from migraine without aura and 48.0% among persons suffering from migraine with aura. Brain health check-up findings of those who had migraine revealed cerebral aneurysm (1.1%) and cerebral arteriovenous malformation (0.6%).3)
In the workplace and brain health check-up, the number of persons affected by migraine is more than 10% higher than the prevalence of migraine reported in other epidemiological surveys.4)5) Despite this high prevalence, the studies have confirmed that appropriate medical care for headache is not being implemented.
Occupational health physicians and brain health check-up physicians should identify serious secondary headaches and promptly refer the affected workers and health check-up receivers to appropriate medical facilities, organize headache educational activities in the workplace to detect persons with primary headaches that cause disability in daily living, and guide these persons to receive appropriate medical care.
• References
1) Von Korff M, Stewart WF, Simon DJ, Lipton RB: Migraine and reduced work performance: a population-based diary study. Neurology 1998; 50(6): 1741-1745.
2) Igarashi H, Sakai F: Survey on the status of migraine in the workplace. Ministry of Health, Labour and Welfare Grants-in-aid for Medical Research on Mental Health “Research Related to the Development of Diagnostic and Treatment Guidelines for Chronic Headache”, Summary and Co-investigator’s Study Reports for 2003. 2004: pp88-91. (In Japanese)
3) Ikeda K, Kashihara H, Hosozawa K, Anan K, Shimomura M, Tamura M, Iwasaki Y, Sakai F: Brain check-up-based study of migraine in Japan. Headache Care 2005; 2(2): 75-80.
4) Sakai F, Igarashi H: Prevalence of migraine in Japan: a nationwide survey. Cephalalgia 1997; 17(1): 15-22.
5) Takeshima T, Ishizaki K, Fukuhara Y, Ijiri T, Kusumi M, Wakutani Y, Mori M, Kawashima M, Kowa H, Adachi Y, Urakami K, Nakashima K: Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache 2004; 44(1): 8-19.
• Search terms and secondary sources
• Search database: PubMed (2011/12/21)
Headache & {Epidemiology} 3263
& {Migraine} 785
& {Japan} 7
CQ I-20
How should school physicians manage headache?
Recommendation
In addition to primary headaches such as migraine and tension-type headache, headaches encountered in schools also include headache as one form of psychosomatic pain. In schools, school nurses look after children who complain of headache, but school physicians are also sometimes consulted regarding headaches. Therefore, school physicians should possess correct knowledge on primary headaches (especially migraine). Headaches may be related to the circumstances surrounding the children, such as stress with teachers and classmates in school or problems at home. Therefore, understanding the background of the children and the mental issues during the developmental process is sometimes necessary.
| Grade A |
Background and Objective
Among the complaints of children at school, headache is one of the most common symptoms. Although headache can be a symptom of acute diseases such as upper respiratory tract infection, primary headaches represented by migraine and tension-type headache are also frequently encountered in school settings. While the teacher in charge of the class is usually the first to deal with headache, the actual care is provided by the school nurse. The school physician provides health consultation for school children and students, and is also consulted about headache through the school nurse during health check-ups and other situations. In students who refuse to go to school or enter classroom, headache is a common reason. Therefore school physicians may also be consulted on psychosocial issues through the school counselor. A literature search was conducted on the management of headache by school physicians
Comments and Evidence
The School Health and Safety Act in Japan (final revision in 2008) stipulates the staffing of school physicians in schools. However, when searching for literature in English language, the search term “school doctor” or “school physician” does not exist. Therefore, it is not possible to compare the school physician system among countries. Instead, relatively abundant literature was identified for “psychologist” as a profession related to children and adolescents. These psychologists probably play similar roles as school counselors in Japan. School counselors in Japan work mainly in public junior high schools, part-time, and the majority hold a certificate of clinical psychotherapist. There are few articles on school physicians in Japan; nevertheless a review article that serves as a useful reference was identified. This article points out that school physicians and school nurses should possess correct knowledge about migraine, and that migraine is fundamentally not a disease with a mental problem and should not be linked unnecessarily to the mental aspect. However, in some cases, it is important to discern psychosocial issues including family environment.1)
• Population-based survey
Children with migraine and tension-type headache reported neck pain, facial and jaw pain, and sleep disorder significantly more frequently than children without headache. Fatigue was more frequent in children with migraine than in children with tension-type headache or children without headache. The number of children visiting the school nurse because of headache did not differ significantly between migraine and tension-type headache. Children with migraine were absent from school significantly more often than children with tension-type headache (aged 7-15 years, Sweden).2) Approximately 6.7% of children experienced severe headache during the previous 12 months, and from the mental health aspect, children with severe headache were 3.2 times more likely than children without severe headache to have difficulties and 2.7 times more likely to have impairment (aged 4-17 years, United States).3) School stressors (harassment by peers, schoolwork pressure, and being treated poorly by teachers) were strongly associated with psychosomatic pain (headache and abdominal pain) as well as psychological symptoms (aged 10-18 years, Sweden).4) Headaches were associated with emotional problems from parent-reported questionnaire, and with general anxiety disorder from child-reported questionnaire (aged 6-11 years, France).5) Frequent headache was significantly associated with teacher unfairness, and classmate social support acted as a protective factor but not as a buffering mechanism (aged 11, 13 and 15 years, Italy).6
• School-based activity study of patients attending headache center of pediatric hospital
School children with headache did not differ from the healthy control children without headache in terms of social goodwill and friendship. Elementary school children with migraine had fewer friends at school, but middle school students with migraine were identified by peers as displaying higher levels of leadership and popularity (aged 8-14 years, United States).7)
• References
1) Teramoto J: Children with chronic diseases and school education. Children with headache and school life. Kyoiku To Igaku 2010; 58(10): 988-996. (In Japanese)
2) Laurell K, Larsson B, Eeg-Olofsson O: Headache in schoolchildren: association with other pain, family history and psycho-social factors. Pain 2005; 119(1-3): 150-158.
3) Strine TW, Okoro CA, McGuire LC, Balluz LS: The associations among childhood headaches, emotional and behavioral difficulties, and health care use. Pediatrics 2006; 117(5): 1728-1735.
4) Hjern A, Alfven G, Ostberg V: School stressors, psychological complaints and psychosomatic pain. Acta Paediatr 2008; 97(1): 112-117.
5) Pitrou I, Shojaei T, Chan-Chee C, Wazana A, Boyd A, Kovess-Masfety V: The associations between headaches and psycho-pathology: a survey in school children. Headache 2010; 50(10): 1537-1548.
6) Santinello M, Vieno A, De Vogli R: Primary headache in Italian early adolescents: the role of perceived teacher unfairness. Headache 2009; 49(3): 366-374.
7) Vannatta K, Getzoff EA, Gilman DK, Noll RB, Gerhardt CA, Powers SW, Hershey AD: Friendships and social interactions of school-aged children with migraine. Cephalalgia 2008; 28(7): 734-743.
• Search terms and secondary sources
• Search database: PubMed (2011/10/2)
Headache 54748 &children 7917
& children adolescents 4900
& (school) 752 OR (doctor) 35 OR (physician) 49 OR (nurse) 14 OR (psychologist) 136
• Search database: Ichushi Web for articles published in Japan (excluding proceedings) (2011/10/16)
School physician 705
& primary school middle school 30
& primary school middle school kindergarten 4
& headache 2
CQ I-21
What are the important points in patient education and doctor‒patient relationship?
Recommendation
As for all disciplines of medical care, good doctor‒patient relationship is necessary to obtain high quality headache care. A headache management program that puts emphasis on patient education improves disability and functional health status, and increases satisfaction. When informing a patient of the accurate diagnosis, the doctor should at the same time explain the appropriate management and treatment of headache to the patient, and educate the patient where necessary.
| Grade A |
Background and Objective
The effectiveness of prophylactic and acute treatments for chronic headache is directly linked to the understanding and appropriate management of headache by the patient him/herself. Patient education is extremely important in headache care. Furthermore, to promote patient education, building a good doctor‒patient relationship is essential.
Comments and Evidence
Several non-controlled studies have shown that patient education program or comprehensive headache treatment program including patient education improves patients’ QOL regardless of the type of headache.1)-11) By explaining to patients with headache regarding the type of headache they have, the mechanisms by which symptoms appear, self-management skills, therapies, medications and their mechanisms of action as well as adverse effects, and instructing them about what to pay attention in daily life, disability and functional health status are improved and satisfaction is increased. Patient education has to be conducted thoroughly. To increase the motivation of patients toward treatment, building a good doctor‒patient relationship is important. Furthermore, studies in recent years have indicated the association between chronicity of migraine and health problems such as sleep12) and obesity.13) Increasing emphasis is being put on education of these research results as well as interventions.
• References
1) Harpole LH, Samsa GP, Jurgelski AE, Shipley JL, Bernstein A, Matchar DB: Headache management program improves outcome for chronic headache. Headache 2003; 43(7): 715-724.
2) Lipton RB, Silberstein SD, Saper JR, Bigal ME, Goadsby PJ: Why headache treatment fails. Neurology 2003; 60(7): 1064-1070.
3) Sheftell FD: Communicating the right therapy for the right patient at the right time: acute therapy. Can J Neurol Sci 2002; 29(Suppl 2): S33-39.
4) Lipton RB, Silberstein SD: The role of headache-related disability in migraine management: implications for headache treatment guidelines. Neurology 2001; 56(6 Suppl 1): S35-42.
5) Ivers H, McGrath PJ, Purdy RA, Hennigar AW, Campbell MA: Decision making in migraine patients taking sumatriptan: an exploratory study. Headache 2000; 40(2): 129-136.
6) Lipton RB, Stewart WF, MacGregor A, et al: Communication of migraine disability between physicians and patients. Cephalalgia 1999; 19: 337.
7) Slater ND, Lipton RB, Stewart WF, et al: Doctor-patient communication about migraine disability. Neurology 1999; 52(suppl 2): A470.
8) Mannix LK, Solomon GD, Kippes CM, Kunkel RS: Impact of headache education program in the workplace. Neurology 1999; 53(4): 868-871.
9) Meisler JG: Patient education. Headache. J Womens Health Gend Based Med 1999; 8(6): 737-738.
10) Lipton RB, Stewart WF, Simon D: Medical consultation for migraine: results from the American Migraine Study. Headache 1998; 38(2): 87-96.
11) MacGregor EA: The doctor and the migraine patient: improving compliance. Neurology 1997; 48(3 Suppl 3): S16-20.
12) Evers S: Headache and sleep: the missing links. Cephalalgia 2011; 31(6): 643-644.
13) Nicholson R, Bigal M: Screening and behavioral management: obesity and weight management. Headache 2008; 48(1): 51-57.
• Search terms and secondary sources
• Search database: PubMed (2012/6/5)
headache.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] & education.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] limited to (review) 260
headache.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] & doctor patient relationship.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] limited to (review) 95
CQ I-22
How to evaluate the medico-economic effect of appropriate treatment for migraine?
Recommendation
In Japan, it is estimated that migraine causes an economic loss of approximately three hundred billion yen per year.1) Compared to traditional migraine medications, proper use of triptan greatly improves patients’ QOL at an acceptable level of increase in medical expenses, and the health benefit leads to reduction in overall cost to the society.
| Grade B |
Background and Objective
In Japan, headache causes an estimated economic loss of approximately six hundred billion yen per year, and migraine contributes to a half of this loss.1) Since triptan is a costly medication, many studies from European and American countries evaluated the cost-effectiveness of triptan in the 1990s. A representative economic assessment of triptan is the Canadian Coordinating Office for Health Technology Assessment (CCOHTA) conducted by a health department. The study concluded that incremental health benefits were obtained from using oral triptan rather than oral ergotamine and that these benefits were achieved at acceptable incremental costs to healthcare resources. When society as a whole was considered, the health benefits also resulted in a net reduction of overall costs to society. In other words, a conclusion has been arrived that triptan is superior in terms of cost-effectiveness. In Japan, an article examining the cost-effectiveness of triptan tablet has been published.
Comments and Evidence
In an assessment conducted by the Canadian health department, from the societal perspective, using sumatriptan instead of caffeine/ergotamine resulted in an incremental cost-effectiveness ratio of 25 Canadian dollars ($Can) per attack suppressed, an increment of $Can7,507 for obtaining each quality-adjusted life-year (QALY), and a net economic benefit to the society of $Can42 per patient per year.2) From the perspective of the insurance payer, the incremental cost-effectiveness ratio was $Can98 per attack suppressed, and the increment was $Can29,366 per QALY. The grade of recommendation based on decisions regarding health technology for adoption into health insurance plans was ‘moderate’. Sensitivity analysis showed that the results were robust despite relatively large changes in the input variables.
In the evaluation conducted by Shimizu et al.,3) avoiding an episode of migraine required an extra economic burden of only approximately 600 yen. In addition, an extra expenditure of more than two million yen is required to live healthily without being troubled by attack for one year. Comparing this extra expenditure with the Canadian insurance reimbursement standard, it is equivalent to a recommendation grade of “moderate”. The authors concluded that sumatriptan tablet for migraine is a treatment with high cost-effectiveness.
In recent years, medication overuse headache due to excessive use of triptan has become a problem. For this reason, there is a concern that this situation will increase medical expenditure and lower labor productivity, consequently lowering the medico-economic value of triptan. Proper use of triptan is an issue that should always be borne in mind.
• References
1) Manaka N, Kitamura T: Department of Economics, University of Headache.
http: //homepage2.nifty.com/uoh/gakubu/keizaigakubu.htm (In Japanese)
2) Evans KW, Boan JA, Evans JL, Shuaib A: Economic evaluation of oral sumatriptan compared with oral caffeine/ergotamine for migraine. Pharmacoeconomics 1997; 12(5): 565-577.
3) Shimizu T, Iwata M, Nishimura S: Economic impact of oral sumatriptan in the treatment of migraine in Japan. Shindan To Shinyaku 2001; 38: 787-799. (In Japanese)
• Search terms and secondary sources
• Search database: Ichushi Web for articles published in Japan (2011/12/21)
migraine/ and medical economics 6, migraine and cost-effectiveness 9
• Search database: PubMed (2011/12/21)
Migraine and cost-effectiveness 162, Migraine and economic evaluation 143, Migraine and pharmacoeconomics
CQ I-23
Is there a need for multidisciplinary team approach to headache treatment?
Recommendation
Despite advances in headache treatment, there remain many patients with chronic headache in whom pharmacotherapy alone is not adequately effective. For the treatment of refractory headache, a multidisciplinary team led by the headache specialist and supported by other health professionals including clinical psychotherapist, physical therapist, occupational therapist, nurse, pharmacist and acupuncturist is essential.
| Grade A |
Background and Objective
Despite the advances in acute treatment and prophylactic therapy for chronic headache, there are still many patients who do not respond adequately to pharmacotherapy alone. A scientific session on multidisciplinary treatment of headache was organized at the European Headache and Migraine Trust International Congress (EHMTIC) in 2010. The session concluded that effective multidisciplinary headache program (MTP) can be expected to reduce the frequency of headache and the disease burden, as well as decrease the risk for medication overuse headache.1 In the future, MTP provided by a headache team led by the headache specialist and supported by other health professionals including clinical psychotherapist, physical therapist, occupational therapist, nurse, pharmacist and acupuncturist is indispensable for the treatment of refractory headache. The Japanese Headache Society has started board certification of headache specialist from 2005, and subsequently headache outpatient clinics began to be established around the country. The challenge ahead will be to educate and train headache specialists and other health professionals specializing in headache treatment.
Comments and Evidence
A nationwide epidemiological survey in Japan estimated that approximately 40 million people suffered from chronic headache, 8.4 million of whom had migraine, and that headache impaired the activities of daily living in 74% of those affected.2) These figures show that despite the recent advances in headache treatment, many patients still do not achieve improvement in symptoms.
Recent reports have indicated that MTP provided by a headache team led by physician and supported by other health professionals from multiple disciplines is essential, and that MTP is effective in alleviating the impairing and disabling effects of chronic headache, and increasing the patients’ level of satisfaction.1)3)-9) The MTP usually involves three disciplines comprising physicians, physical therapists and psychotherapists,4)5) or four disciplines with the addition of nurses.6)-9) In the headache school of the MTP, the team participants work together to educate patients with chronic headache about the diagnosis of headache, acute treatment, prophylactic treatment, risk factors and mechanisms of medication overuse headache, and implementation of non-pharmacological prophylactic treatment strategies (Figure 1).1) The physician is responsible for performing neurological examinations on patients with chronic headache, excluding secondary headache, establishing the correct diagnosis, prescribing pharmacotherapy, and at the same time playing a leading role in deciding the therapy plans within the team.1) The physical therapist evaluates the musculoskeletal system, and verifies the effectiveness of various interventions such as exercise therapy, exercise for relief of headache, massage, and hot pack.1)10) The psychotherapist implements cognitive-behavioral therapy and is sometimes essential as a bridge to the psychiatrist or psychosomatic physician.1)10) The roles of the headache nurse include taking a headache history from patients with chronic headache, listening to their complaints or anxiety, obtaining information on individual and social background, and providing technical guidance on self-injection of sumatriptan at home. Acupuncture has been reported to be effective in the prevention of migraine.11) Although evidence is currently inadequate, trial of this approach is worthwhile. Instructions provided by the pharmacist regarding taking of the prescribed medications is expected to increase the level of satisfaction of patients with chronic headache, and improve the therapeutic effect. At the Saitama International Headache Center, occupational therapists analyze the patients’ headache diaries in detail by conducting interviews with the patients.10) Lemstra et al.4) assigned migraine patients to MTP (n = 44) or non-MTP group (n = 36) for six weeks, and observed significant improvements in headache frequency, headache intensity, quality of life, and depression in the MTP group, at the end of intervention and after 3 months. Gunreben-Stempfle et al.5) reported that a 96-hour MTP was more effective than a 20-hour program. Zeeberg et al.6) showed that MTP reduced headache intensity, headache frequency, and headache-related absence from work for headaches other than post-traumatic headache, while Jensenet al.7) reported that female gender, migraine, and triptan overuse predicted good outcome from MTP. Gaul et al.9) conducted a four-discipline MTP for 5 days in 295 patients with primary headache, and reported that the mean headache frequency decreased from 13.4 to 8.8 days per month after 12-18 months, and that 43% of the subjects accomplished the primary outcome which was 50% reduction of headache frequency.
From the above findings, MTP implemented by a headache team is undoubtedly essential for the treatment of chronic headache. However, the methodology lacks adequate scientific evidence, and further discussion is therefore necessary. In recent years, there is an increase in nurses specializing in chronic diseases, such as Japanese Nursing Association-certified nurses in dementia nursing and certified nurses in diabetes nursing. There is also a need for the training of nurses and other health professionals specializing in headache treatment.
Figure 1. All disciplines contribute to the headache school.
[Reproduced from Gaul C, Visscher CM, Bhola R, Sorbi MJ, Galli F, Rasmussen AV, Jensen R: Team players against headache: multidisciplinary treatment of primary headaches and medication overuse headache. J Headache Pain 2011; 12(5): 511-519, under the Creative Commons Attribution license]
• References
1) Gaul C, Visscher CM, Bhola R, Sorbi MJ, Galli F, Rasmussen AV, Jensen R: Team players against headache: multidisciplinary treatment of primary headaches and medication overuse headache. J Headache Pain 2011; 12(5): 511-519.
2) Sakai F, Igarashi H: Prevalence of migraine in Japan: a nationwide survey. Cephalalgia 1997; 17(1): 15-22.
3) Harpole LH, Samsa GP, Jurgelski AE, Shipley JL, Bernstein A, Matchar DB: Headache management program improves outcome for chronic headache. Headache 2003; 43(7): 715-724.
4) Lemstra M, Stewart B, Olszynski WP: Effectiveness of multidisciplinary intervention in the treatment of migraine : a randomized clinical trial. Headache 2002; 42(9): 845-854.
5) Gunreben-Stempfle B, Griessinger N, Lang E, Muehlhans B, Sittl R, Ulrich K: Effectiveness of an intensive multidisciplinary headache treatment program. Headache 2009; 49(7): 990-1000.
6) Zeeberg P, Olesen J, Jensen R: Efficacy of multidisciplinary treatment in a tertiary referral headache centre. Cephalalgia 2005; 25(12): 1159-1167.
7) Jensen R, Zeeberg P, Dehlendorff C, Olesen J: Predictors of outcome of the treatment programme in a multidisciplinary headache centre. Cephalalgia 2010; 30(10): 1214-1224.
8) Magnusson JE, Riess CM, Becker WJ: Effectiveness of a multidisciplinary treatment program for chronic daily headache. Can J Neurol Sci 2004; 31(1): 72-79.
9) Gaul C, van Doorn C, Webering N, Dlugaj M, Katsarava Z, Diener HC, Fritsche G: Clinical outcome of a headache-specific multidisciplinary treatment program and adherence to treatment recommendations in a tertiary headache center : an observational study. J Headache Pain 2011; 12(4): 475-483.
10) Sakai F. Headache treatment system (headache specialist, headache clinic, medical collaboration). Chiryo 2011; 93(7): 1609-1613. (In Japanese)
11) Linde K, Allais G, Brinkhaus B, Manheimer E, Vickers A, White AR: Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev 2009; 21(1): CD001218.
• Search terms and secondary sources
• Search database: PubMed (2012/4/30)
{headache} & {team play} 7
{headache} & {multidisciplinary treatment} 193
{headache} & {management program} 269
• Search database: Ichushi Web for articles published in Japan
headache outpatient 142
headache outpatient clinic 12
headache center 28
headache specialist 7
• Secondary source: 2 additional references from manual search (references 2 and 11)
CQ I-24
How is headache or facial or neck pain attributed to cervical carotid or vertebral artery dissection diagnosed?
Recommendation
• Headache or facial or neck pain attributed to cervical carotid or vertebral artery dissection is new, acute-onset headache, with facial or neck pain, usually unilateral (ipsilateral to dissecting artery), and severe.
• The pain of vertebral artery dissecting aneurysm is mostly localized in the back of the head or the neck, whereas pain due to internal carotid artery dissection occurs commonly in the front of the head or the forehead.
• The pain is persistent, but resolves within one month.
• The modes of onset can be classified broadly into ischemic (cerebral infarction, transient ischemic attack), hemorrhagic (subarachnoid hemorrhage), and others (headache, local symptoms, others).
• For diagnosis, while cerebral angiography is essential for a definitive diagnosis, noninvasive imaging techniques such as MRI, magnetic resonance angiography (MRA), and three-dimensional CT angiography (3D-CTA) are useful and provide important imaging information especially on dissection.
| Grade A |
Background and Objective
Approximately 70% of the patients with dissecting aneurysm of the internal carotid artery or cervical artery have headache. In recent years, with increasing attention given to this disease due to widespread use of noninvasive diagnostic imaging techniques such as MRI and MRA, the opportunity of detection has also increased. The natural course of this disease is good in most patients. However, in some cases, the clinical state changes greatly in the early stage, with rebleeding and brainstem ischemia that may result in serious sequelae or even death. The objective of this section is to describe differential diagnosis by physicians attending the patients in the early stage.
Comments and Evidence
In the International Classification of Headache Disorders, 3rd edition (beta version) of the International Headache Society, this disease is classified as 6.5.1 “Headache or facial or neck pain attributed to cervical carotid or vertebral artery dissection”.1) The diagnostic criteria are as follows:
A. Any new headache and/or facial or neck pain fulfilling criterion C
B. Cervical carotid or vertebral dissection has been diagnosed
C. Evidence of causation demonstrated by at least two of the following:
1. pain has developed in close temporal relation to other local signs of cervical artery dissection, or has led to the diagnosis of cervical artery dissection
2. either or both of the following:
a) pain has significantly worsened in parallel with other signs of the cervical artery lesion
b) pain has significantly improved or resolved within 1 month of its onset
3. either or both of the following:
a) pain is severe and continuous for days or longer
b) pain precedes signs of acute retinal and/or cerebral ischemia
4. pain is unilateral and ipsilateral to the affected cervical artery
D. Not better accounted for by another ICHD-3 diagnosis.
Sudden and severe headache or neck pain is an important characteristic of artery dissection. The frequency of headache or neck pain associated with dissection has been reported to be 60 to 80%. Headache or neck pain arises due to dissection of the vascular wall, and is considered to be referred pain along the blood vessel. In the vertebral artery territory, distribution of the 2nd and 3rd spinal nerves gives rise to pain in the back of the head and the neck. In the internal carotid artery territory, distribution of the trigeminal nerve often gives rise to pain in the front of the head and the forehead. It should be noted that there is no specific pattern of headache onset, as is also commented in the International Headache Classification. This condition is often misdiagnosed as other headaches including migraine, cluster headache, and primary thunderclap headache.2)-10) Since a suspicion of this condition as well as early diagnosis and treatment are vital, MRI, MRA and 3D-CTA should be conducted. Furthermore, performing conventional angiography is important to confirm a definitive diagnosis. In addition, since cerebral aneurysm is an important cause of cerebral infarction in young adults, the possibility of dissecting cerebral aneurysm should always be borne in mind in younger stroke patients aged below 50 years.11) In recent years, cervical artery dissection manifesting headache or neck pain only has received attention. In cases with mild lumen formation, the natural course is favorable. However, some cases progress to serious conditions such as subarachnoid hemorrhage and cerebral infarction.12) At present, evidence-based treatment for cases manifesting headache or neck pain only has not been established.13)14)
For further studies of headache and pain associated with dissecting cerebral aneurysm, cases should be accumulated to examine the characteristics not only for the vertebrobasilar artery territory but also for other territories of the internal carotid artery, anterior cerebral artery, and middle cerebral artery.15)-18)
• References
1) Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33(9): 629-808.
2) Biousse V, D’Anglejan-Chatillon, Massiou H, Bousser MG: Head pain in non-traumatic artery dissection: a series of 65 patients. Cephalalgia 1994; 14(1): 33-36.
3) Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG: Time course of symptoms in extracranial carotid artery dissection. A series of 80 patients. Stroke 1995; 26(2): 235-239.
4) Biousse V, Woimant F, Amarenco P, Touboul PJ, Bousser MG: Pain as the only manifestation of internal carotid artery dissection. Cephalalgia 1992; 12(5): 314-317.
5) de la Sayette V, Leproux F, Letellier P: Cervical cord and dorsal medullary infarction presenting with retro-orbital pain. Neurology 1999; 53(3): 632-634.
6) Duffy RF, Snijders CJ, Vannesle JA: Spontaneous bilateral internal carotid artery dissection and migraine: a potential diagnostic delay. Headache 1997; 37(2): 109-112.
7) Guillon B, Biousse V, Massiou H, Bousser MG: Orbital pain as an isolated sign of internal carotid artery dissection. A diagnostic pitfall. Cephalalgia 1998; 18(4): 222-224.
8) Nakatomi H, Nagata K, Kawamoto S, Shiokawa Y: Ruptured dissecting aneurysm as a cause of subarachnoid hemorrhage of unverified etiology. Stroke 1997; 28(6): 1278-1282.
9) Ramadan NM, Tietjen GE, Levine SR, Welch KM: Scintillating scotomata associated with internal carotid artery dissection: report of three cases. Neurology 1991; 41(7): 1084-1087.
10) Silbert PL, Mokri B, Schievink WI: Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology 1995; 45(8): 1517-1522.
11) Suyama K, Hayashi K, Nagata I: Cervicocephalic arterial dissection. Brain and Nerve 2008; 60(10): 1115-1123. (In Japanese with English abstract)
12) Yagi S, Yoshioka H, Yagi T, Ohashi Y, Nakano S, Ogawa M, Fukamachi A: Management of intracranial vertebral artery dissections presenting with isolated pain. Surg Cereb Stroke 2005; 33(1): 14-19. (In Japanese with English abstract)
13) Shinohara Y, Ogawa A, Suzuki N, Katayama Y, Kimura A (Eds.): Japanese Guidelines for the Management of Stroke 2009. Kyowa Kikaku, 2009. (In Japanese)
14) Ono J, Hirai S, Serizawa T, Kobayashi S, Kobayashi E, Sugaya Y, Karasudani H, Ozawa Y, Saeki N, Yamaura A: Treatment strategy of non-hemorrhagic arterial dissection in vertebrobasilar system. Surg Cereb Stroke 2005; 33(1): 20-25. (In Japanese with English abstract)
15) Ono J, Higuchi Y, Matsuda S, Fujikawa A, Machida T, Okiyama K, Nagano O, Aoyagi K, Kobayashi E, Saeki N, Yamaura A: Clinical features and treatment strategy in intracranial arterial dissection of ischemic onset. Surg Cereb Stroke 2011; 39(4): 272-277. (In Japanese with English abstract)
16) Ono J, Higuchi Y, Saeki N, Yamaura A: V. Significance and interpretation of nationwide survey. 23. Significance and interpretation of nationwide survey on cerebral artery dissection. Saito I, Hashi K (Supervising Editor), Inoue T (Editor): The Mt. Fuji Workshop on CVD Vol. 29 Artery Dissection and Stroke. Neuron Corporation, 2011, pp. 117-121. (In Japanese)
17) Watanabe M, Tanaka T, Ota N, Nakatogawa H, Kenmochi H, Chimura M. Koizumi S, Fujimoto A, Inenaga C, Hiramatsu H, Yamamoto T, Sugiura Y, Namba H, Hanakita J, Sakai T: Significance of the characteristics of precedent headache and nuchal pain associated with non-traumatic cerebral dissecting aneurysms in the posterior fossa: an investigation of 57 consecutive cases at our institution. Jpn J Neurosurg 2011; 20(5): 381-391. (In Japanese)
18) Tamakawa N, Ishikawa T, Moroi J, Yoshioka S, Muto T, Hikichi K, Fukui K, Yamashita S, Kobayashi S, Sasaki M, Suzuki A, Yasui N: I. Vertebro-basilar artery dissection (presentation and diagnosis) 7. Study of headache associated with cerebral dissecting aneurysm: what kind of pain is the pain associated with dissection. The Mt. Fuji Workshop on CVD Vol. 29 Artery Dissection and Stroke. Neuron Corporation, 2011, pp. 26-28. (In Japanese)
• Search terms and secondary sources
• This search (2012/4/10)
• PubMed: Headache & Dissecting cerebral aneurysm = 197 articles, Dissecting cerebral aneurysm & Diagnosis & Clinical Features = 76 articles
• Ichushi Web for articles published in Japan
Dissecting cerebral aneurysm = 248 articles
• Note 1
The diagnostic criteria for cerebral artery dissection have been reported by the Cerebral and Cardiovascular Disease Commissioned Study Group in Japan. They are shown below for reference.
1. Vascular lumen findings
a. Either intimal flap or double lumen observed on cerebral angiography
b. Intimal flap or double lumen observed on CTA images
c. Hyperintensity suggesting intramural hematoma on T1-weightd MRI
d. Findings suggesting artery dissection (dilatation and stenosis, retention of the contrast media, string sign, tapered occlusion) on cerebral angiography
e. Dilatation and stenosis observed on MRA or CTA
f. Intimal flap or double lumen observed on MRI, MRA or contrast-enhanced T1-weighted MRI
g. Fusiform dilatation in the main branch of artery observed on angiography, MRA or CTA
2. Arterial surface appearance findings
Surface appearance of artery showing fusiform dilatation on contrast-enhanced (volume) T1-weighted image or basiparallel anatomic scanning (BPAS) or 3D-T2-weighted MRI.
3. Change in imaging finding during follow-up
Definitive changes (reduced or augmented) of 1 or 2 on follow-up images.
4. Surgical and histopathological findings
a. Artery dissection observed during surgery
b. Histopathological examination of resected or autopsied specimen showing cerebral artery dissection
[Definite dissection]
When one of the following three applies:
• Among a, b and c of 1 above, one is fulfilled
• 3 above is fulfilled and causes other than dissection are excluded
• For 4 above, either a or b is fulfilled
[Probable dissection]
When one of the following three applies:
• For 1 above, either d or e is fulfilled
• Either f of 1 above or 2 is fulfilled
• Stenosis or obstruction is observed in artery, and 2 is fulfilled
[Possible dissection]
• Either f and g of 1 above, or 2 is fulfilled
[Tsukahara T: Status of cerebral artery dissection (1) Outline of disease state, symptoms, diagnosis and treatment of cerebral artery dissection. Guidance for examination of cerebral artery dissection. Cardiovascular Disease Study Commission Grant 18 Ko-5 (SCADS-Japan) Disease state of cerebral artery dissection and treatment development (principal investigator: Minematsu K) Cerebrovascular Division, Department of Internal Medicine, National Cerebral and Cardiovascular Center 2009: pp 1-7.]
• Note 2
Descriptions related to cerebral artery dissection in the Japanese Guideline for the Management of Stroke are shown below:
II. Cerebral infarction/transient ischemic attack (TIA)
2. Treatment of cerebral infarction caused by special conditions
2-1. Cerebral artery dissection
For cerebral infarction caused by cerebral artery dissection, select treatment method for individual cases according to the degree of vascular stenosis and aneurysm formation (grade C1)
2-2. Aorta dissection
For cerebral infarction complicating aortic dissection, intravenous alteplase therapy is contraindicated (grade D).
VI. Other cerebrovascular diseases
1. Intra-/extra-cranial artery dissection
1-1. Medical treatment for intra-/extra-cranial artery dissection (anti-thrombotic therapy)
1. For extracranial cervical artery dissection with onset of ischemic symptoms, antithrombotic therapy (anticoagulant therapy or antiplatelet therapy) should be considered in the acute stage (grade C1).
2. For intracranial artery dissection with onset of ischemia, antithrombotic therapy (anticoagulant therapy or antiplatelet therapy) may be considered in the acute stage (grade C1). However, since intracranial dissection may have a risk of subarachnoid hemorrhage, antithrombotic therapy should be withheld if aneurysm formation is clearly observed in the dissecting site (grade C2).
3. To prevent recurrence in cases of cerebral artery dissection with onset of ischemia, antithrombotic therapy (anticoagulant therapy or antiplatelet therapy) should be considered. Since the findings in the dissection site change over time, conduct imaging examination every three months, and consider change or continuation of antithrombotic therapy based on the imaging findings (grade C1).
1-2. Surgical treatment for intra-/extra-cranial artery dissection
1. For hemorrhagic cerebral artery dissection, early diagnosis and treatment are recommended because of the high risk of rebleeding (grade C1). When surgical treatment is selected, conducting surgery within 24 hours of bleeding is recommended (grade C1).
2. For nonhemorrhagic cerebral artery dissection, conservative treatment is usually selected if the natural history is unknown. In that case, regular follow-up by MRI or angiography is recommended (grade C1).
3. Direct surgery and endovascular treatment both have advantages and disadvantages. Evaluate the indication individually (grade C1). Direct surgery is useful when revascularization is necessary. On the other hand, endovascular treatment is less invasive and treatment can be started earlier, and is frequently selected as the surgical treatment (grade C1). From the viewpoint of preventing rebleeding, trapping of the lesion site is recommended. If trapping is difficult, consider proximal occlusion of the parent artery (grade C1).
[Shinohara Y, Ogawa A, Suzuki N, Katayama Y, Kimura A (Eds.) Japanese Guidelines for the Management of Stroke 2009. Kyowa Kikaku, 2009, quoted and abstracted from p. 74, 75, 244 and 247]
CQ I-25
How is headache attributed to spontaneous intracranial hypotension diagnosed and treated?
Recommendation
1. Diagnosis
Headache attributed to spontaneous intracranial hypotension is diagnosed according to the International Classification of Headache Disorders 3rd Edition beta version (ICHD-3beta). Confirmation of cerebrospinal fluid leak by diagnostic imaging is important. The ICHD-3beta does not indicate the criteria for diagnostic imaging; therefore diagnosis should use the guidelines proposed by the Japanese Ministry of Health, Labour and Welfare Study Group (published in October 2011) as reference.
| Grade B |
2. Treatment
Conservative treatments such as bed rest and fluid infusion should be conducted. When there is no improvement and if the site of cerebrospinal fluid leak can be identified by diagnostic imaging, invasive treatments such as epidural blood patch should be considered.
| Grade A |
Background and Objective
According to the International Classification of Headache Disorders 3rd Edition beta version (ICHD-3beta), headache attributed to low cerebrospinal fluid pressure is coded under 7 “Headache attributed to non-vascular intracranial disorder” type 7.2 “Headache attributed to low cerebrospinal fluid pressure”, and is further classified into the following subforms1):
7.2.1 “Post-dural puncture headache”
7.2.2 “CSF fistula headache”
7.2.3 “Headache attributed to spontaneous intracranial hypotension”
Previously used terms for headache attributed to spontaneous intracranial hypotension include “spontaneous low CSF pressure; ICHD second edition (ICHD-II)”, “primary intracranial hypotension”, “low CSF-volume headache”, and “hypoliquorrhoeic headache”. In the ICHD-3beta, 7.2.3 “Headache attributed to spontaneous intracranial hypotension” was adopted.1)
Headache attributed to spontaneous intracranial hypotension is considered to be fundamentally caused by a loss in cerebrospinal fluid volume.1)-5) Although cerebrospinal fluid hypovolemia can give rise to diverse symptoms, the core symptom is orthostatic headache. According to the Monro-Kellie doctrine, cerebrospinal fluid pressure is compensated and becomes normalized.5) Therefore, the disease name “cerebrospinal fluid hypovolemia” has been advocated for headache attributed to spontaneous intracranial hypotension.6)
Despite having the word “spontaneous” in the disease name, recently several etiologies have been proposed for headache attributed to spontaneous intracranial hypotension, such as leak from the dural sleeve that passes through the nerve root (dural tear) and leak from meningeal diverticulum.4)-6) The triggers include straining, coughing, drastic lowering of atmospheric pressure, sexual activity, craniocervical injury, falling on the rear, and dura weakness due to abnormal connective tissue. Note that other causes of low cerebrospinal fluid pressure may exist, including reduced production of cerebrospinal fluid due to vitamin A deficiency.7)
Reports from Japan have shown that “cerebrospinal fluid hypovolemia” may be included among cases diagnosed as post-head injury sequel, whiplash injury, autonomic ataxia, general malaise, chronic fatigue syndrome, and depression.6)8)
Comments and Evidence
In the ICHD-II,1) the diagnostic criteria for 7.2.3 “headache attributed to spontaneous low CSF pressure” are as follows:
A. Diffuse and/or dull headache that worsens within 15 minutes after sitting or standing, with at least one of the following and fulfilling criterion D:
1. neck stiffness
2. tinnitus
3. hypacusia
4. photophobia
5. nausea
B. At least one of the following:
1. evidence of low CSF pressure on MRI (eg, pachymeningeal enhancement)
2. evidence of CSF leak on conventional myelography, CT myelography or cisternography
3. CSF opening pressure <60 mm H2O in sitting position
C. No history of dural puncture or other cause of CSF fistula
D. Headache resolves within 72 hours after epidural blood patching
In the recently published ICHD-3beta,2) the diagnostic criteria for 7.2.3 “headache attributed to spontaneous intracranial hypotension” are described below:
A. Any headache fulfilling criterion C
B. Low CSF pressure (<60 mm CSF) and/or evidence of CSF leakage on imaging
C. Headache has developed in temporal relation to the low CSF pressure or CSF leakage, or has led to its discovery
D. Not better accounted for by another ICHD-3 diagnosis.
As shown above, the ICHD-II criteria provide concise definitions for the symptoms, test findings and treatments for headache attributed to spontaneous low CSF pressure [hereinafter referred to as spontaneous intracranial hypotension: SIH]. For the diagnosis and treatment of SIH, it is appropriate to start from these diagnostic criteria. Criterion D concerns symptom improvement after blood epidural blood patch. However, this does not imply that headache attributed to spontaneous low CSF pressure cannot be diagnosed without conducting a blood patch. This criterion should be interpreted as “headache resolves within 72 hours in the case that blood patching is conducted for SIH”.
After publication of the ICHD-II, renowned researchers from the United States proposed new criteria as the basis for change in future revision of the classification criteria.9) The proposed diagnostic criteria are shown in Table 1. A characteristic of these criteria is that the time requirement was eliminated. Subsequently, in the ICHD-3beta published in 2013, the time factor described in the ICHD-II has been removed, as shown above.
Table 1. Diagnostic criteria for headache due to spontaneous intracranial hypotension.
| A. Orthostatic headache |
| B. The presence of at least one of the following: |
| 1. Low opening pressure (≤ 60 mmH2O) |
| 2. Sustained improvement of symptoms after epidural blood patching |
| 3. Demonstration of an active spinal CSF leak |
| 4. Cranial MRI changes of intracranial hypotension (eg, brain sagging or pachymeningeal enhancement) |
| C. No recent history of dural puncture |
| D. Not attributable to another disorder |
[Schievink WI, Dodick DW, Mokri B, Silberstein S, Bousser MG, Goasdsby PJ: Diagnostic criteria for headache due to spontaneous intracranial hypotension: a perspective. Headache 2011; 51(9): 1442-1444.]
Headache
The typical headache is orthostatic headache. However, cases of unremarkable orthostatic headache, or paradoxically rare cases of postural headache,4) and cases manifesting thunderclap headache9) have been reported. Most patients experience orthostatic headache at some point during the disease course. Apart from spontaneous intracranial hypotension syndrome, other causes of orthostatic headache such as postural orthostatic tachycardia syndrome (POTS)11) have to be included in the differential diagnosis.
Symptoms other than headache
The ICHD-II listed other symptoms such as neck stiffness, tinnitus, hypacusia, photophobia, and nausea. The symptoms of cerebrospinal fluid hypovolemia described by the Japanese Cerebrospinal Fluid Hypovolemia Study Group are presented in Table 2. These symptoms are exacerbated by a mild state of dehydration such as fever and diarrhea.6) In the proposed criteria for future revision mentioned above,9) symptoms other than orthostatic headache included in the ICHD-II were deleted (Table 1). Subsequently, in the ICHD-3beta, accompanying symptoms have been removed from the diagnostic criteria, but the description carries a sentence that “It is usually accompanied by neck stiffness and subjective hearing symptoms”.
Table 2. Symptoms of cerebrospinal fluid hypovolemia (Cerebrospinal Fluid Hypovolemia Study Group).
| (1) Major symptoms | |
| Headache, neck pain, vertigo, tinnitus, visual disturbance, weariness/fatigability | |
| (2) Accompanying symptoms) | |
| 1. Cranial nerve symptoms | blurred vision, nystagmus, oculomotor palsy (pupil dilation, ptosis of eyelid), diplopia, photophobia, visual field disturbance, facial pain, facial numbness, hearing loss, abducens palsy, facial palsy, hypacusia |
| 2. Nerve dysfunction other than cranial nerve symptoms | Impaired consciousness, apathy, cerebella ataxia, gait disturbance, Parkinson syndrome, dementia, dysmnesia, radiculopathy, pain/numbness of upper extremity, vesicorectal disturbance, etc. |
| 3. Endocrinologic abnormality | Galactorrhoea, etc. |
| 4. Others | Nausea/vomiting, neck stiffness, interscapular pain, lumbar pain, etc. |
[Guideline Committee of Cerebrospinal Fluid Hypovolemia Study Group (Ed.): Cerebrospinal fluid hypovolemia guideline 2007 (in Japanese). Cited and abstracted from p. 16, 2007]
Cerebrospinal fluid pressure
For the diagnosis of SIH, although it is important to perform a lumbar puncture to prove low cerebrospinal fluid pressure, the lumbar puncture per se may elicit further cerebrospinal fluid leak. Therefore, in patients with already positive MRI findings such as pachymeningeal enhancement, lumbar puncture should be performed upon consideration of its necessity for treatment. In SIH also, the cerebrospinal fluid pressure may be normalized according to Monro-Kellie doctrine (Miyazawa5) and Mokri et al.12) both reported normal pressure in 18%).
Diagnostic imaging
The modalities of diagnostic imaging for cerebrospinal fluid hypovolemia include radionuclide (RI) cisternography for detecting cerebrospinal fluid leak, CT/MR myelography and spine MRI for obtaining direct findings, and cranial MRI for detecting indirect findings due to reduced cerebrospinal fluid. Table 3 summarizes the imaging modalities examined in many reports.4)-6) Conventional CT has little diagnostic value. Occasionally, spontaneous intracranial hypotension syndrome is complicated by bilateral chronic subdural hematomas. In this case, CT would help the diagnosis. Pachymeningeal enhancement on MRI is a strong evidence for a suspicion of spontaneous intracranial hypotension syndrome. However, this finding is not always depicted. On the other hand, pachymeningeal enhancement is observed in many diseases including dura invasion of malignant tumor and hypertrophic pachymeningitis, and exclusion of these conditions is necessary.5)
Table 3. Image diagnostic criteria for cerebrospinal fluid hypovolemia.
| (1) Findings of low cerebrospinal fluid pressure (indirect finding) |
| MRI [plain + gadolinium enhancement, sagittal + coronal] |
| (a) Brain shift finding |
| Enlargement of subdural space, descent of amygdala, disappearance of suprasellar cistern, flattening of brainstem (pons) |
| (b) Congestion findings |
| Diffuse pachymeningeal enhancement, dilation of superficial veins of the brain, enlargement of pituitary gland |
| (2) Diagnosis of cerebrospinal fluid leak (direct findings) |
| RI cisternography, CT/MR myelography, spinal MRI |
| (a) Cerebrospinal fluid leak finding |
| (3) Diagnosis of cerebrospinal fluid leak (indirect findings) |
| RI cisternography |
| (a) Early renal uptake of RI |
| (b) Increased RI clearance |
| (c) Cerebrospinal fluid circulatory failure |
In recent years, to solve the confusion over the disease concept and diagnostic criteria of headache attributed to spontaneous low CSF pressure (spontaneous intracranial hypotension syndrome) and cerebrospinal fluid hypovolemia, which has become a social problem, a research project funded by the Japanese Ministry of Health, Labour and Welfare Grant-in-aid for Scientific Research on the “Establishment of Diagnosis and Treatment of Cerebrospinal Fluid Hypovolemia (principal investigator: Kayama Takamasa)” was started in 2007. This Study Group published the “Guidelines for diagnosis and treatment of cerebrospinal fluid leak” in October 2011,13) which was approved by the Japan Neurosurgical Society, Japanese Society of Neurology, the Japanese Orthopaedic Association, the Japanese Headache Society, the Japan Society of Neurotraumatology, Japanese Society of Spinal Surgery, The Japanese Society for Spine Surgery and Related Research, and Japan Medical Society of Spinal Cord Lesion. The Study Group reasoned that “even if the pathological condition of ‘loss of cerebrospinal fluid volume’ advocated by Mokri et al. does exist, the volume of cerebrospinal fluid cannot be measured clinically. At this point in time, the only diagnoses possible are ‘intracranial hypotension’ and ‘cerebrospinal fluid leak’”. Based on this rationale, the Study Group first developed the criteria to diagnosis cerebrospinal fluid leak (Table 4). Given that cerebrospinal fluid leak is closely related to intracranial hypotension, the diagnostic criteria for spontaneous intracranial hypotension syndrome were also published (Table 5). The patients diagnosed according to these criteria are eligible for the advanced medical care (blood patch) which was approved for health insurance in June 2012 (to be described below). For this guideline, the detailed image diagnostic criteria are published elsewhere,13) and are not provided here due to space limitation.
Table 4. Image diagnostic criteria for cerebrospinal fluid leak (partially abstracted).
| Image diagnosis of cerebrospinal fluid leak |
| “Definitive” finding |
| CT myelography: |
| Finding of epidural leak of contrast medium continuous with the subarachnoid space |
| “Probable” finding |
| CT myelography: |
| Finding of epidural leak of contrast medium not continuous with the puncture site |
| Spinal MRI/MR myelography |
| Unenhanced epidural water signal lesion continuous with the subarachnoid space |
| RI cisternography: |
| Unilateral localized abnormal RI uptake + cerebrospinal fluid circulatory failure |
| “Strongly suspected” finding |
| Spinal MRI/MR myelography: |
| (1) Unenhanced epidural water signal lesion |
| (2) Epidural water signal lesion continuous with subarachnoid space |
| RI cisternography: |
| (1) Unilateral localized abnormal RI uptake |
| (2) Asymmetrical abnormal RI uptake or symmetrical uptake from neck to chest region, + cerebrospinal fluid circulatory failure |
| “Suspected” finding |
| Spinal MRI/MR myelography: |
| Epidural water signal lesion |
| RI cisternography: |
| (1) Asymmetrical abnormal RI uptake |
| (2) Symmetrical uptake from neck to chest region |
[Sato S, Kayama T: Guidelines for Diagnosis and Treatment of Cerebrospinal Fluid Leak. Noshinkei Geka Sokuho 2012; 22(2): 200-206 (in Japanese)]
Table 5. Diagnostic criteria for spontaneous intracranial hypotension.
[Sato S, Kayama T: Guidelines for Diagnosis and Treatment of Cerebrospinal Fluid Leak. Noshinkei Geka Sokuho 2012; 22(2): 200-206. (in Japanese)]
Cerebrospinal fluid leak (CSF leak) is a disease already included in the International Classification Diseases (ICD-10). Moreover, in a paper published in 2008, Schievink from the United States also advocated that the term cerebrospinal fluid leak should be used because “the underlying cause is a spontaneous spinal cerebrospinal fluid (CSF) leak”.
Treatment
Mokri3) described the treatments for SIH as shown in Table 6.
Table 6. Treatment methods for spontaneous intracranial hypotension syndrome (Mokri, 2004).
| 1. Bed rest | 8. Epidural blood patch |
| 2. Hydration/over-hydration | 9. Continuous epidural saline infusion |
| 3. Caffeine | 10. Epidural infusion of dextran |
| 4. Theophylline | 11. Epidural injection of fibrin glue |
| 5. Abdominal binder | 12. Intrathecal fluid infusion |
| 6. Corticosteroids | 13. Surgical repair of the leak |
| 7. Anti-inflammatory analgesic |
[Mokri B: Spontaneous intracranial hypotension spontaneous CSF leaks. Headache Currents 2005; 2(1): 11-22.]
The treatments for SIH are divided into conservative treatments and invasive treatments. SIH may remit spontaneously. Conservative treatments such as bed rest and fluid infusion (1,000-1,500 mL/day) are effective, and treatment for approximately 2 weeks is recommended.5)6) Invasive treatments include the so called blood patch (epidural blood patch; EBP).4)-8) If the leak site is identified, epidural blood patching is conducted from near the leak.
Previously this procedure was not covered by health insurance. However, advanced medical care (Ministry of Health, Labour and Welfare Notification No. 379-63, Epidural blood patch) for patients fulfilling the diagnostic criteria proposed by the above-mentioned Study Group was approved for health insurance since June 2012. The approved procedure is described below.
(1) The patient is placed in a lateral or prone position on the operating table.
(2) An epidural needle of around 17G is used to perform an epidural puncture, using the loss of resistance method.
(3) Autologous blood is prepared by collecting approximately 15-30 mL of venous blood. 4-10 mL of contrast medium is added for monitoring the injecting area during injection.
(4) Injection is performed under fluoroscopic guidance.
(5) After treatment, the patient bed rests for 1-7 days, and is then discharged.
The efficacy of blood patching has been reported. According to Sencakova et al.,15) 36% (9/25 patients) responded well to the first blood patch, 33% (5/15 patients) became asymptomatic after the second blood patch, and 50% (4/8 patients) responded well after 3 or more (4 on average) blood patch procedures. For traumatic spontaneous intracranial hypotension syndrome, 65% (95/147 patients) achieve improvement or better outcome.7) However, since the diagnostic criteria of the disease are still being debated, precise evaluation of the efficacy of blood patch is a future subject of research.
• References
1) Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24(Suppl 1): 9-160.
2) The Headache Classification Committee of International Headache Society: International Classification of Headache Disorders 3rd Edition beta version 2013. Cephalalgia 33 (9): 716-717.
3) Mokri B: Spontaneous cerebrospinal fluid leaks: from intracranial hypotension to cerebrospinal fluid hypovolemia: evolution of a concept. Mayo Clin Proc 1999; 74(11): 1113-1123.
4) Mokri B: Spontaneous intracranial hypotension spontaneous CSF leaks. Headache Currents 2005; 2(1): 11-22.
5) Miyazawa K: Diagnosis and treatment of spontaneous intracranial hypotension syndrome. No To Shinkei 2004; 56(1): 34-40. (In Japanese)
6) Kitamura T. Spontaneous intracranial hypotension syndrome (cerebrospinal fluid hypovolemia). Kongetsu No Chiryo 2005; 13(5): 549-553. (In Japanese)
7) Teramoto J: [Recent topic on headache] Botulinum toxin and blood patch. No To Shinkei 2004; 56(8): 663-668. (In Japanese)
8) Shinonaga M, Suzuki S: Diagnosis and treatment of traumatic spontaneous intracranial hypotension syndrome (cerebrospinal fluid hypovolemia). Shinkei Gaisho 2003; 26(2): 98-102. (In Japanese)
9) Schievink WI, Dodick DW, Mokri B, Silberstein S, Bousser MG, Goasdsby PJ: Diagnostic criteria for headache due to spontaneous intracranial hypotension: a perspective. Headache 2011; 51(9): 1442-1444.
10) Famularo G, Minisola G, Gigli R: Thunderclap headache and spontaneous intracranial hypotension. Headache 2005; 45(4): 392-393.
11) Mokri B, Low PA: Orthostatic headaches without CSF leak in postural tachycardia syndrome. Neurology 2003; 61(7): 980-982.
12) Mokri B, Hunter SF, Atkinson JL, Piepgras DG: Orthostatic headaches caused by CSF leak but with normal CSF pressures. Neurology 1998; 51(3): 786-790.
13) Sato S, Kayama T: Guidelines for diagnosis and treatment of cerebrospinal fluid leak. Noshinkei Gekka Sokuho 2012; 22(2): 200-206. (In Japanese)
14) Schievink WI: Spontaneous spinal cerebrospinal fluid leaks. Cephalalgia 2008; 28(12): 1345-1356.
15) Sencakova D, Mokri B, McClelland RL: The efficacy of epidural blood patch in spontaneous CSF leaks. Neurology 2001; 57(10): 1921-1923.